Section B Alkanes and cycloalkanes 烷烃与环烷烃
Section B Alkanes and cycloalkanes 烷烃与环烷烃

Key Notes in this chapter B1 Alkanes B2 Cycloalkanes B3 Three dimension structure Conformation
Key Notes in this chapter B1 Alkanes B2 Cycloalkanes B3 Three dimension structure Conformation

重点 EST ABF 必1.烷烃与环烷烃命名 2.烷烃的结构特征 3.立体结构表示方法 4.环烷烃结构特征
重点 1.烷烃与环烷烃命名 2.烷烃的结构特征 3.立体结构表示方法 4.环烷烃结构特征
B1 Definition Alkanes are organic molecules consisting solely of carbon and hydrogen atoms linked by single o bonds. >All the carbon atoms are tetrahedral and sp3 hybridized. >Alkanes are stable molecules and unreactive to most chemical reagents. >Saturated hydrocarbons,straight chain or acyclic carbons Cycloalkanes are cyclic alkane structures. >They have the general formula CnH2n. >Most cycloalkanes are unreactive to chemical reagents
B1 Definition Alkanes are organic molecules consisting solely of carbon and hydrogen atoms linked by single σ bonds. ¾All the carbon atoms are tetrahedral and sp3 hybridized. ¾Alkanes are stable molecules and unreactive to most chemical reagents. ¾Saturated hydrocarbons,straight chain or acyclic carbons Cycloalkanes are cyclic alkane structures. ¾They have the general formula C n H2n. ¾Most cycloalkanes are unreactive to chemical reagents
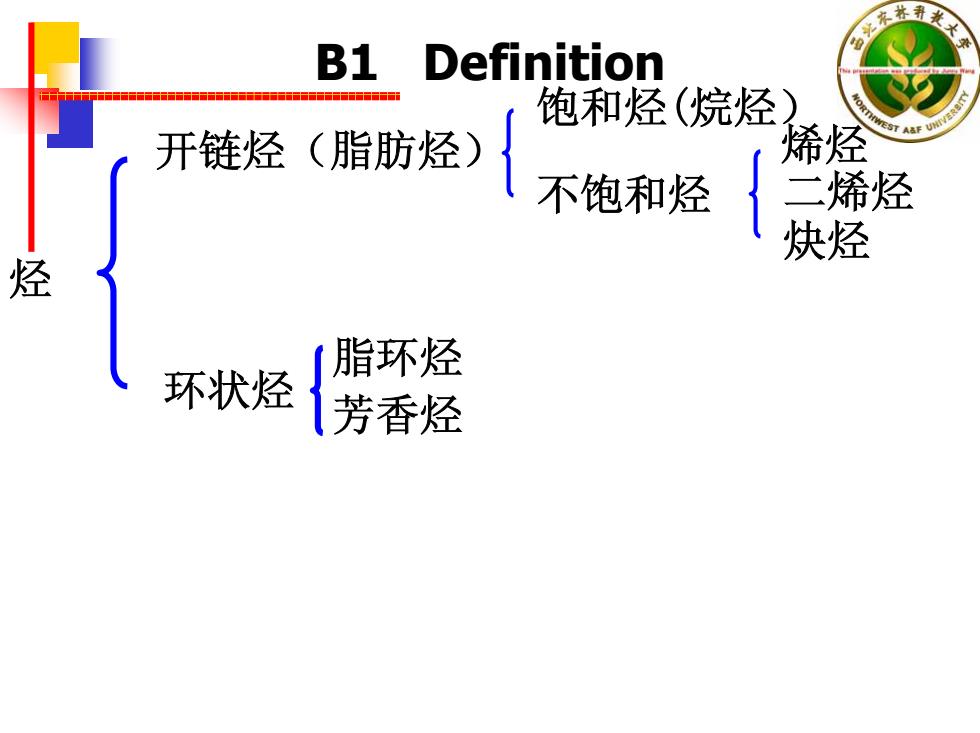
衣林 B1 Definition 饱和烃(烷烃 开链烃(脂肪烃) 烯烃 不饱和烃 二烯烃 炔烃 烃 脂环烃 环状烃 芳香烃
烃 开链烃(脂肪烃) 饱和烃(烷烃) 不饱和烃 烯烃 二烯烃 炔烃 环状烃 脂环烃 芳香烃 B1 Definition
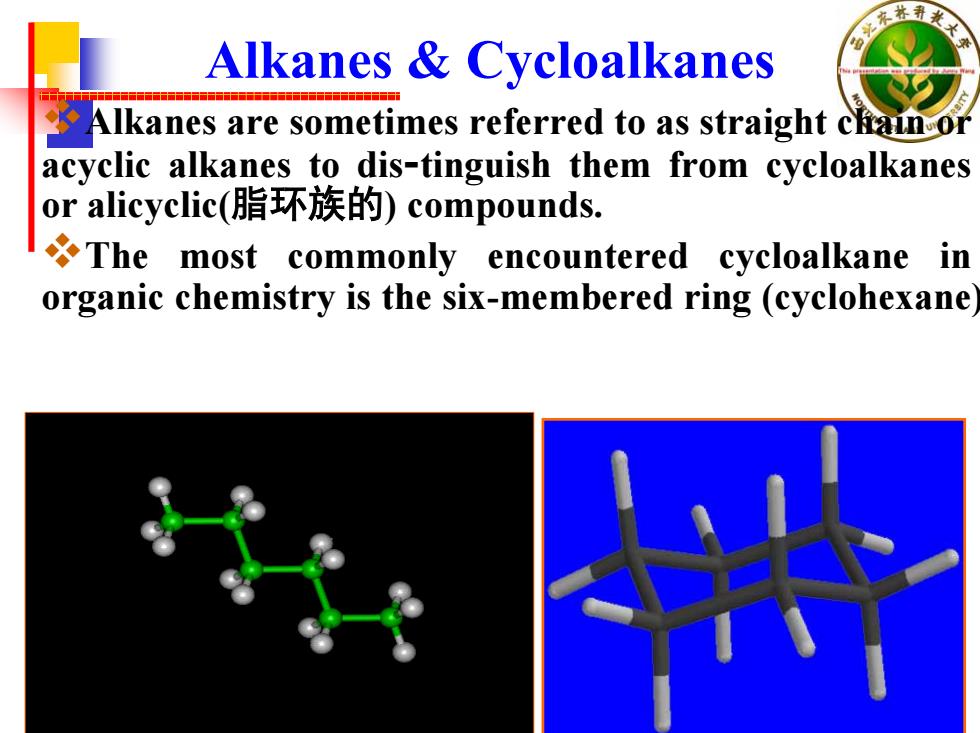
Alkanes Cycloalkanes Alkanes are sometimes referred to as straight chain or acyclic alkanes to dis-tinguish them from cycloalkanes or alicyclic(脂环族的)compounds. The most commonly encountered cycloalkane in organic chemistry is the six-membered ring (cyclohexane
Alkanes & Cycloalkanes Alkanes are sometimes referred to as straight chain or acyclic alkanes to dis-tinguish them from cycloalkanes or alicyclic(脂环族的) compounds. The most commonly encountered cycloalkane in organic chemistry is the six-membered ring (cyclohexane)

Alkanes Cycloalkanes 十十 small three-and four-membered rings are reactive and behave like alkenes. Such cyclic structures are highly strained since it is impossible for the carbon atoms to adopt their preferred tetrahedral shape
Alkanes & Cycloalkanes small three- and four-membered rings are reactive and behave like alkenes. Such cyclic structures are highly strained since it is impossible for the carbon atoms to adopt their preferred tetrahedral shape
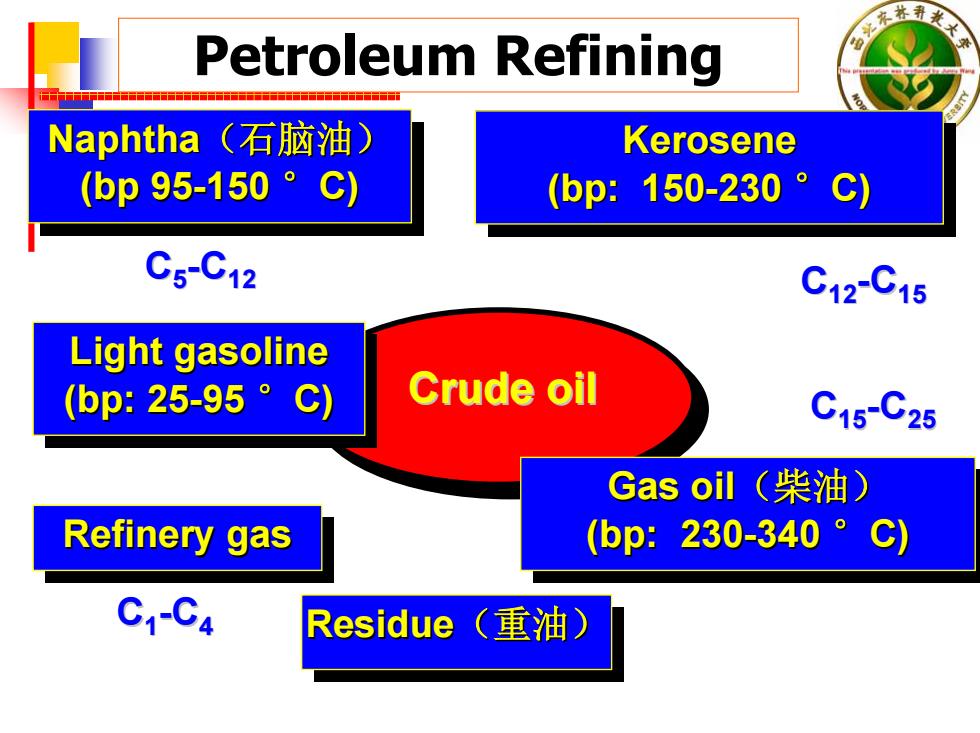
东林 Petroleum Refining Naphtha(石脑油) Kerosene (bp95-150°c) (bp:150-230。C) C5-C12 C12C15 Light gasoline (bp:25-95。C) Crude oil C15-C25 Gas oil(柴油) Refinery gas (bp:230-340。C) C1-C4 Residue (重油)
Petroleum Refining Naphtha(石脑油) (bp 95-150 °C) Naphtha Naphtha (石脑油 ) (bp 95 -150 °C) Kerosene (bp: 150-230 °C) Kerosene Kerosene (bp: 150 -230 °C) Crude oil Crude oil Refinery gas Refinery gas Refinery gas Light gasoline (bp: 25-95 °C) Light gasoline Light gasoline (bp: 25 -95 °C) C 5 - C12 C12 - C15 Gas oil(柴油) (bp: 230-340 °C) Gas oil Gas oil (柴油) (bp: 230 -340 °C) C15 - C25 C 1 - C 4 Residue Residue Residue ((重油) 重油)
林 Petroleum Refining Cracking >converts high molecular weight hydrocarbons to more useful,low molecular weight ones Reforming >increases branching of hydrocarbon chains >branched hydrocarbons have better burning characteristics for automobile engines
Petroleum Refining Cracking ¾converts high molecular weight hydrocarbons to more useful, low molecular weight ones Reforming ¾increases branching of hydrocarbon chains ¾branched hydrocarbons have better burning characteristics for automobile engines

B2 Stereostructure Drawing 立体结构 与立体结构表示方法
B2 Stereostructure Drawing 立体结构 与立体结构表示方法