
有机化学 Organic chemistry 2010 For Undergraduates in biological science 王俊儒张继文袁茂森 应用化学系416,417室 87092187(0) By Junru Wang Email:wangjr07@163.com 西北农林科技大学理学院
Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 6 Stereochemistry :

Content Molecular Chirality Chiral carbon Absolute and Relative Configuration Stereoisomers without chiral carbons Properties of Enantiomers Diastereomers Resolution of Enantiomers

Key Notes Stereochemistry Chiral enantiomers Chirality Chiral center Optical rotations R/S,D/L Diastereomers meso forms Resolution of enantiomers
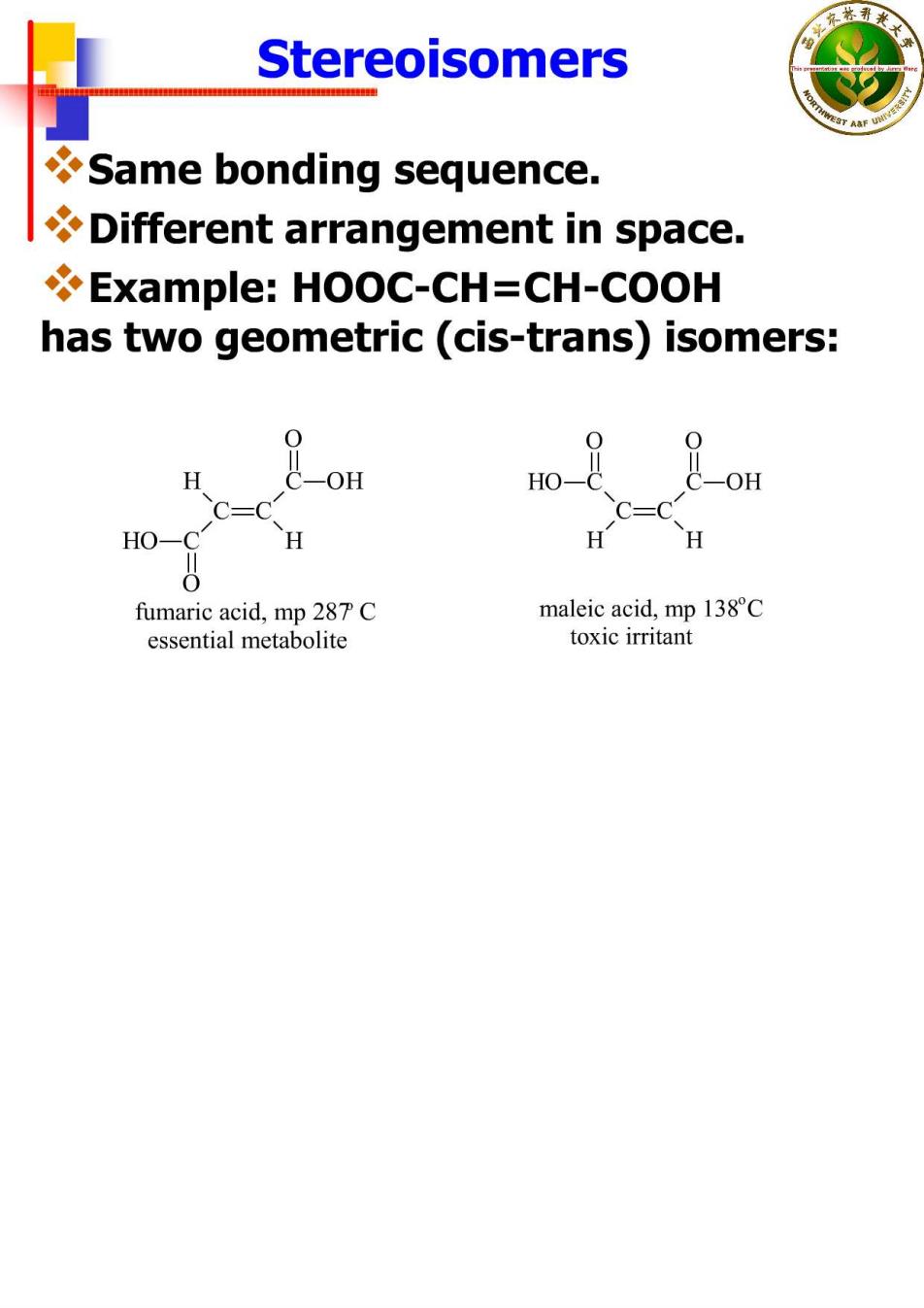
Stereoisomers Same bonding sequence. Different arrangement in space. Example:HOOC-CH=CH-COOH has two geometric(cis-trans)isomers: OH HO- OH HO-( fumaric acid,mp 287 C maleic acid,mp 138C essential metabolite toxic irritant
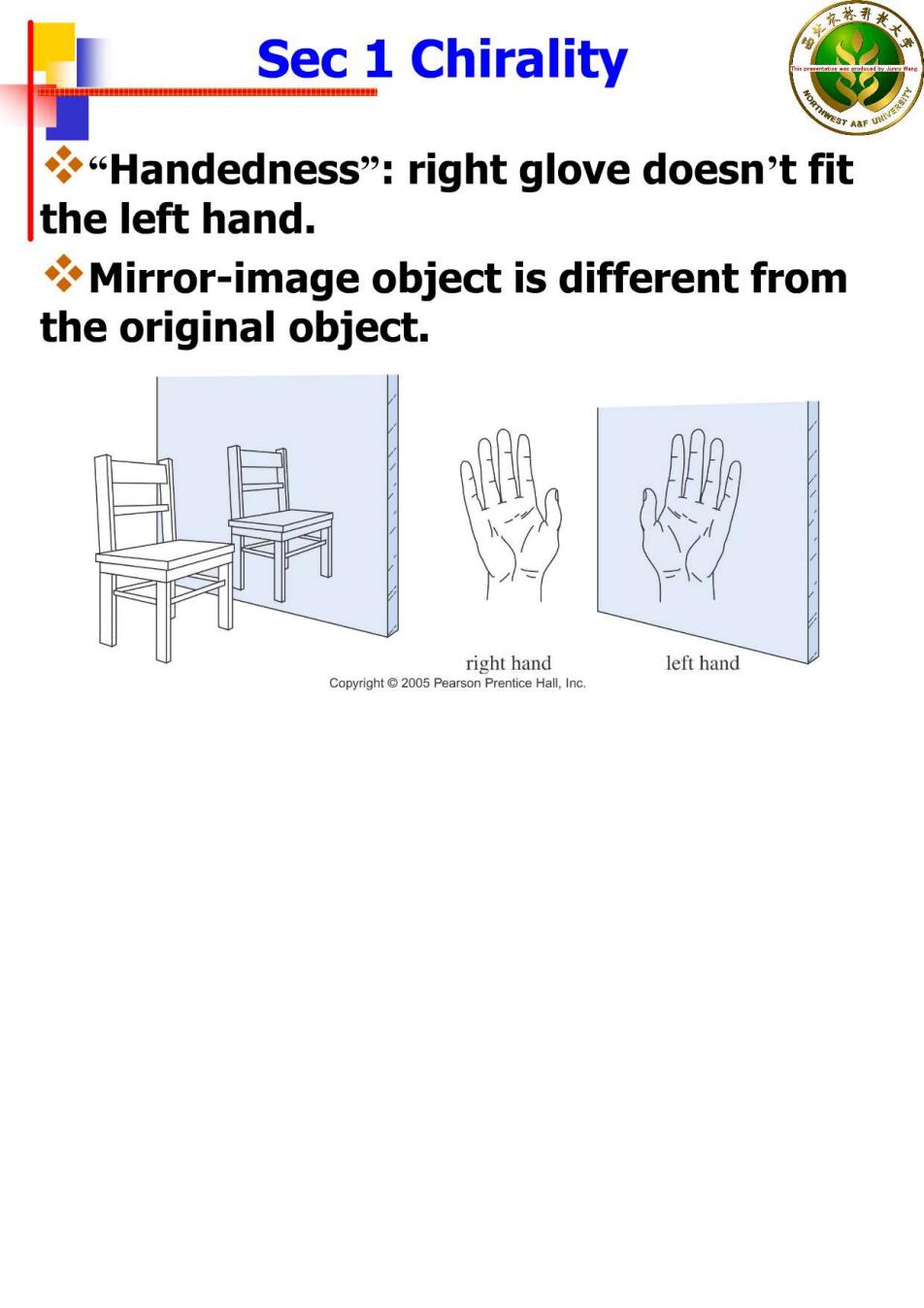
Sec 1 Chirality "Handedness":right glove doesn't fit the left hand. Mirror-image object is different from the original object. right hand left hand Prenice Hall,,lnc
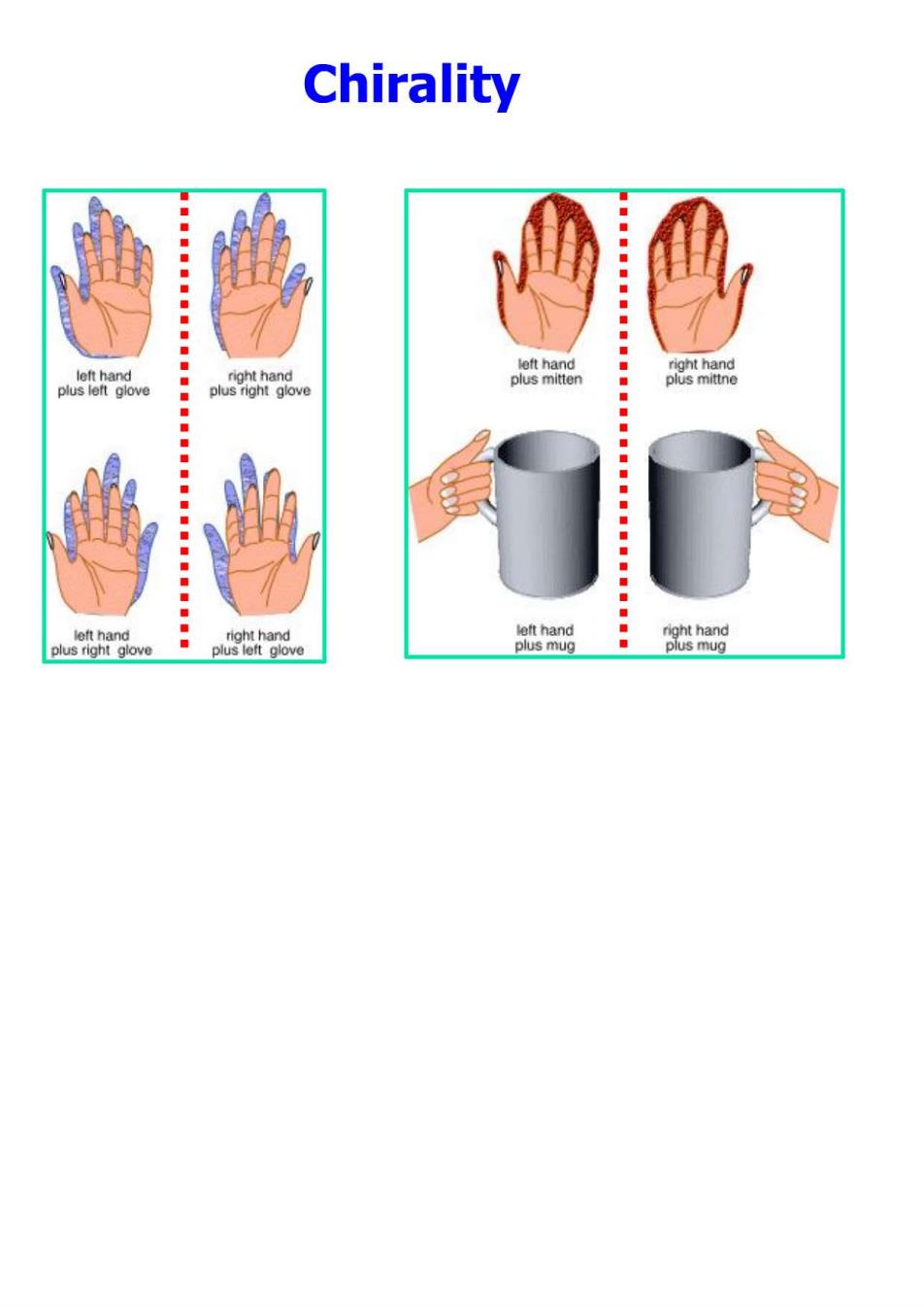
Chirality p8h2g%e

Chirality A molecule is chiral if its two mirror image forms are not superposable upon one another. A molecule is achiral if its two mirror image forms are superposable
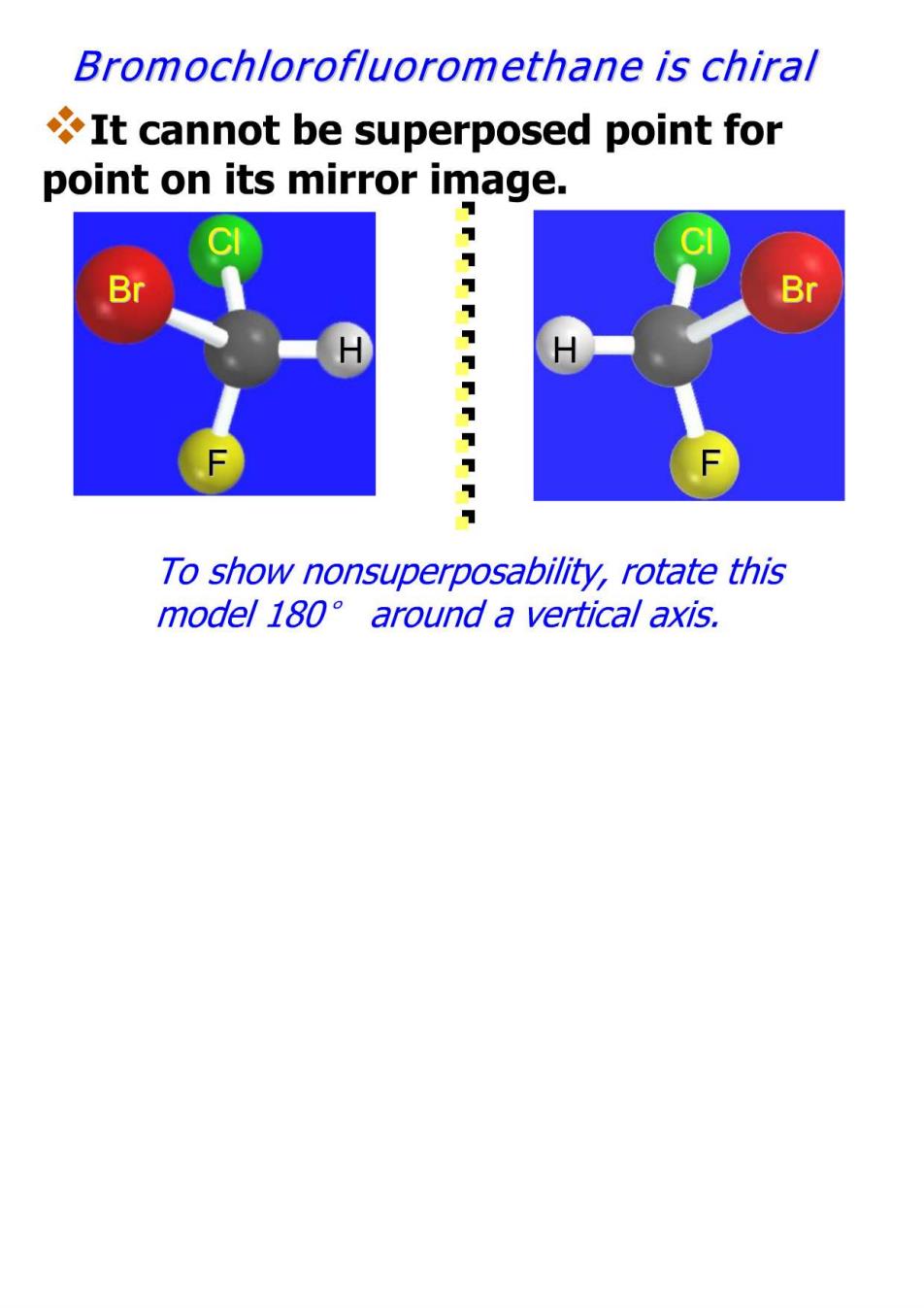
Bromochlorofluoromethane is chiral It cannot be superposed point for point on its mirror image. S F To show nonsuperposability,rotate this model 180 around a vertical axis
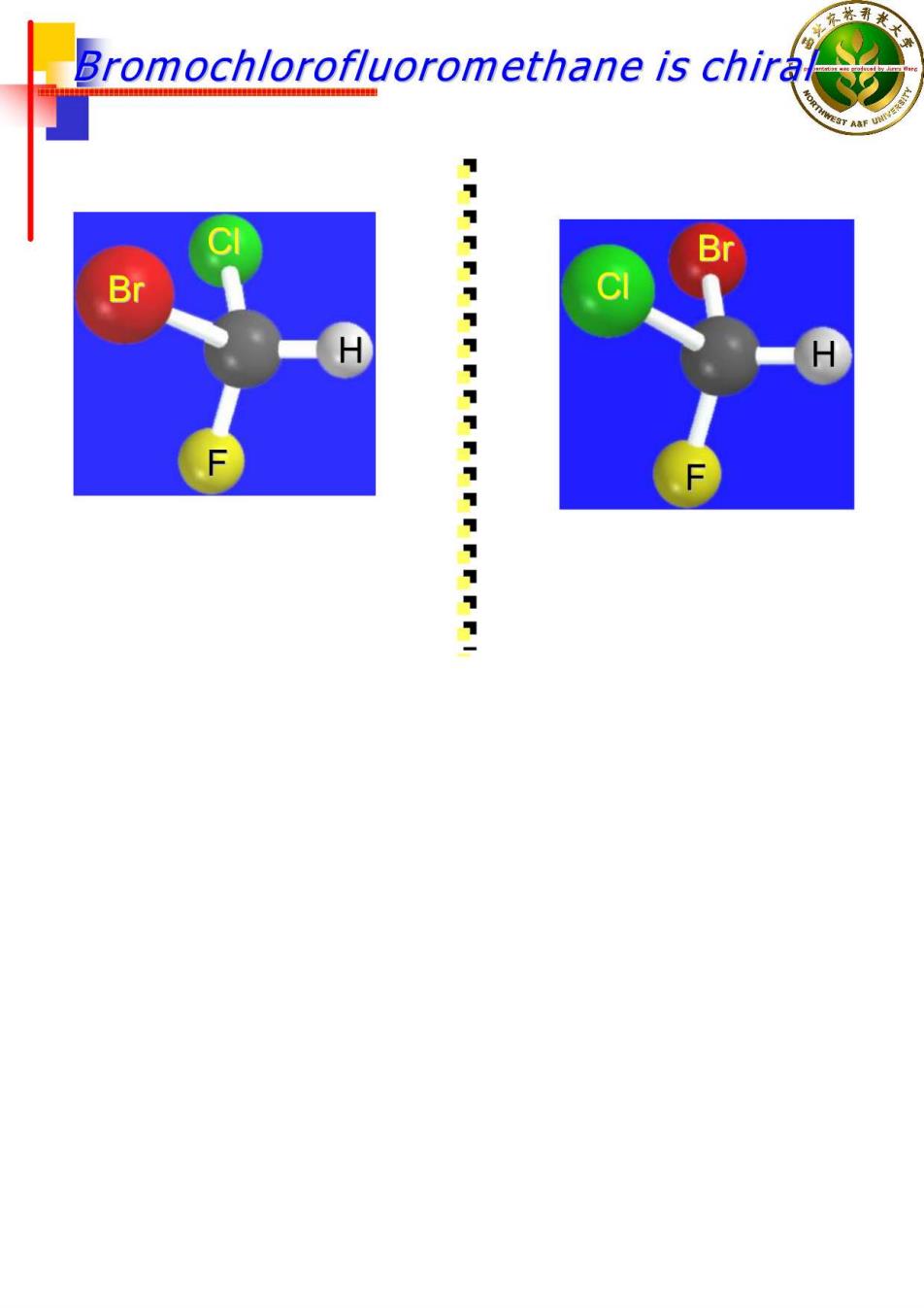
Bromochlorofluoromethane is chira B B C H F 1111111111111111111