东林
College of Science
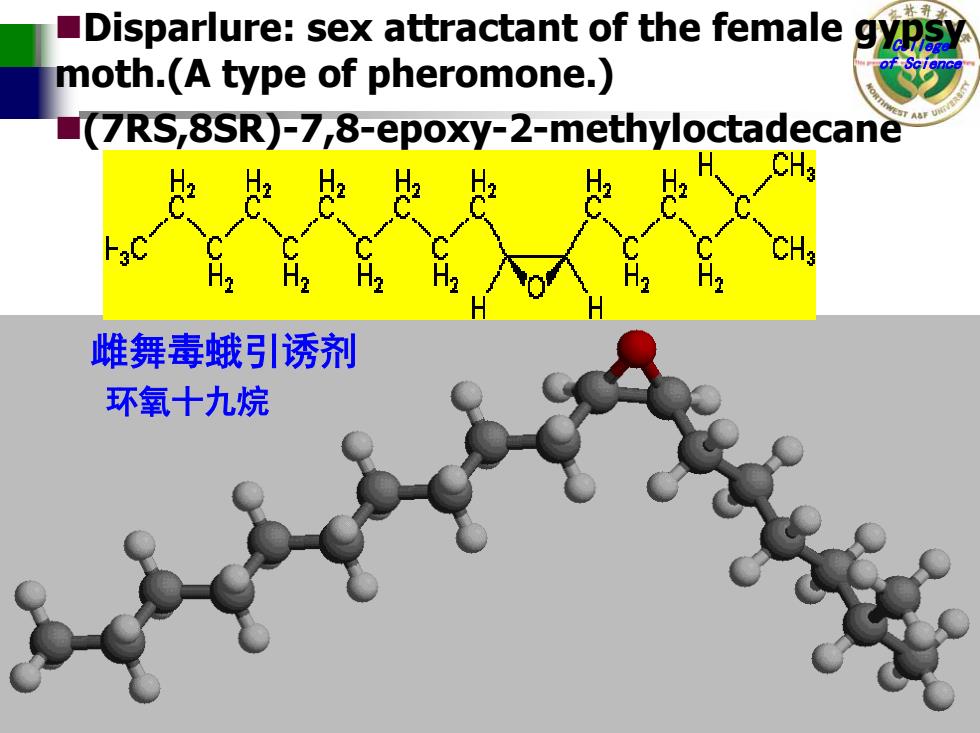
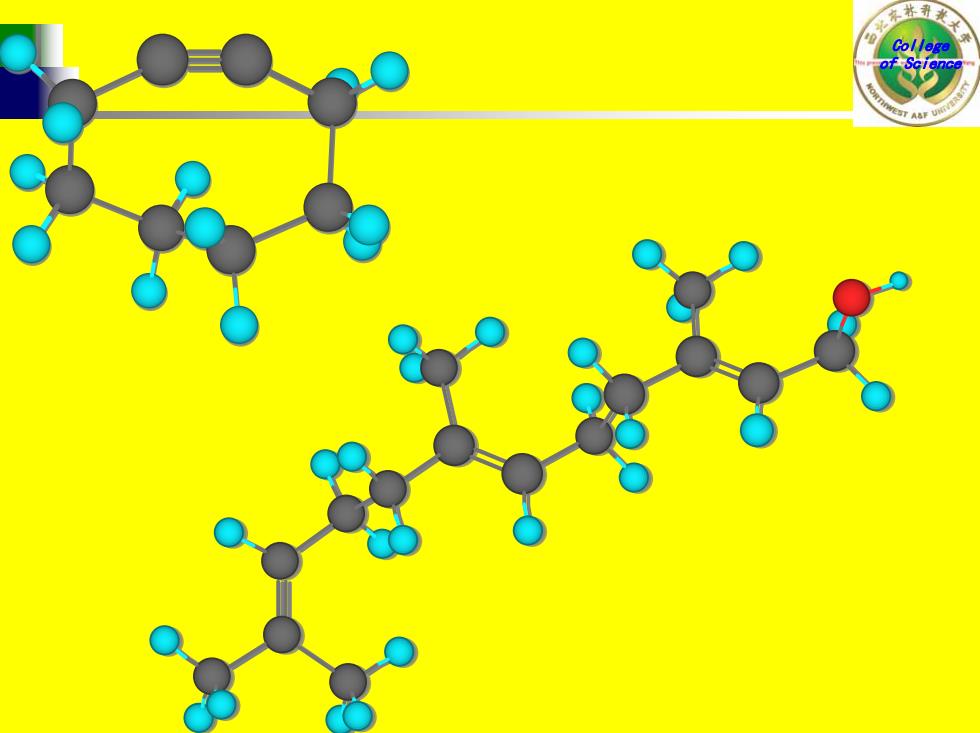
Disparlure:sex attractant of the female moth.(A type of pheromone.) (7RS,8SR)-7,8-epoxy-2-methyloctadecane 2 H 雌舞毒蛾引诱剂 环氧十九烷
College of Science 雌舞毒蛾引诱剂 环氧十九烷 Disparlure: sex attractant of the female gypsy moth.(A type of pheromone.) (7RS,8SR)-7,8-epoxy-2-methyloctadecane
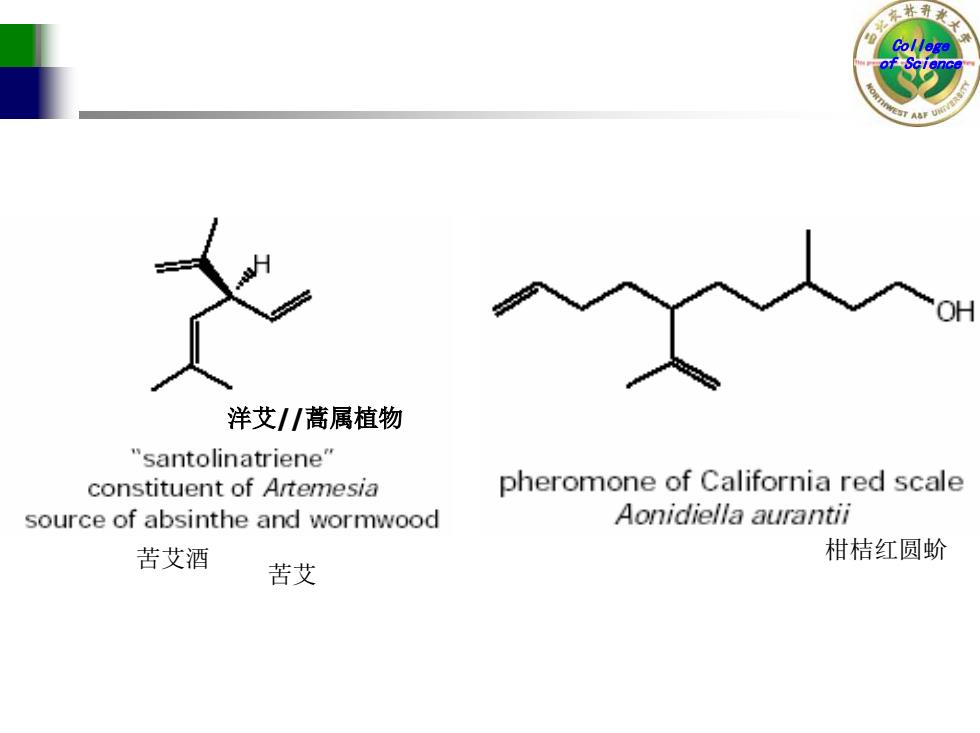
洋艾/蒿属植物 "santolinatriene" constituent of Artemesia pheromone of California red scale source of absinthe and wormwood Aonidiella aurantii 苦艾酒 柑桔红圆蚧 苦艾
College of Science 洋艾//蒿属植物 苦艾酒 苦艾 柑桔红圆蚧

Section H Alkenes and Alkyne ■Preparation ■Properties ■Reactions of alkenes Electrophilic addition to symmetrical alkenes addition to unsymmetrical alkenes Carbocation stabilization Reduction and oxidation of alkenes .Hydroboration of alkenes ■Reactions of alkynes Electrophilic additions to alkynes ●Reduction of alkynes Special reactions of terminal alkynes ■Conjugated dienes
College Section H Alkenes and Alkynesof Science Preparation Properties Reactions of alkenes zElectrophilic addition to symmetrical alkenes zaddition to unsymmetrical alkenes zCarbocation stabilization zReduction and oxidation of alkenes zHydroboration of alkenes Reactions of alkynes zElectrophilic additions to alkynes zReduction of alkynes zSpecial reactions of terminal alkynes Conjugated dienes
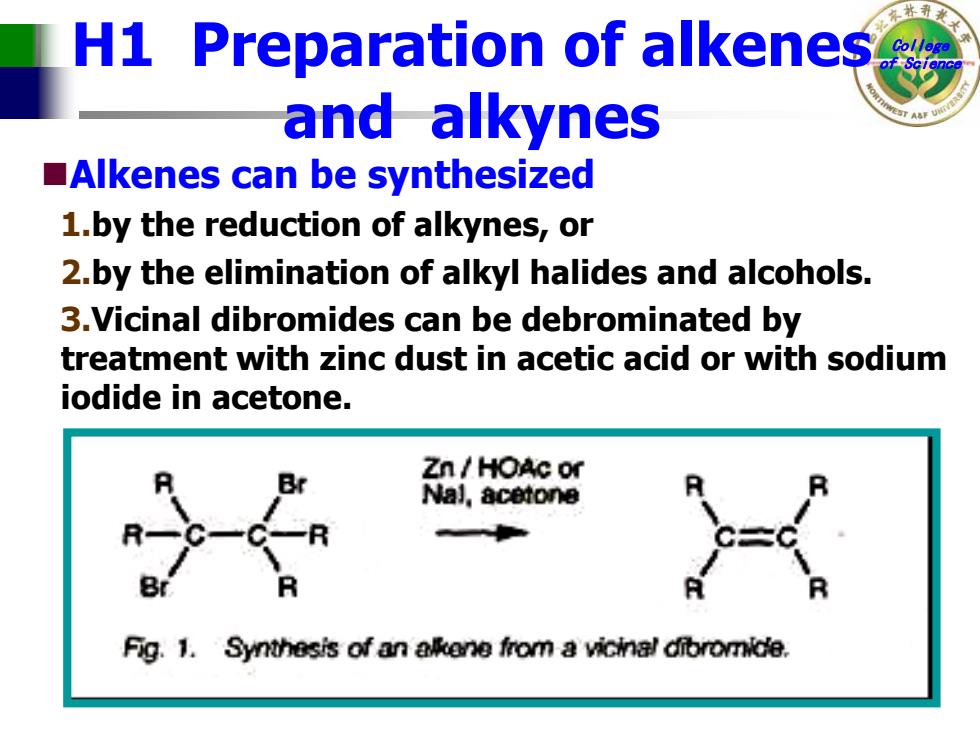
H1 Preparation of alkenes and alkynes Alkenes can be synthesized 1.by the reduction of alkynes,or 2.by the elimination of alkyl halides and alcohols. 3.Vicinal dibromides can be debrominated by treatment with zinc dust in acetic acid or with sodium iodide in acetone. Zn/HOAc or Nal,acetone Fig.1.Synthesis of an alkene from a vicinal dibromide
College H1 Preparation of alkenes of Science and alkynes Alkenes can be synthesized 1.by the reduction of alkynes, or 2.by the elimination of alkyl halides and alcohols. 3.Vicinal dibromides can be debrominated by treatment with zinc dust in acetic acid or with sodium iodide in acetone
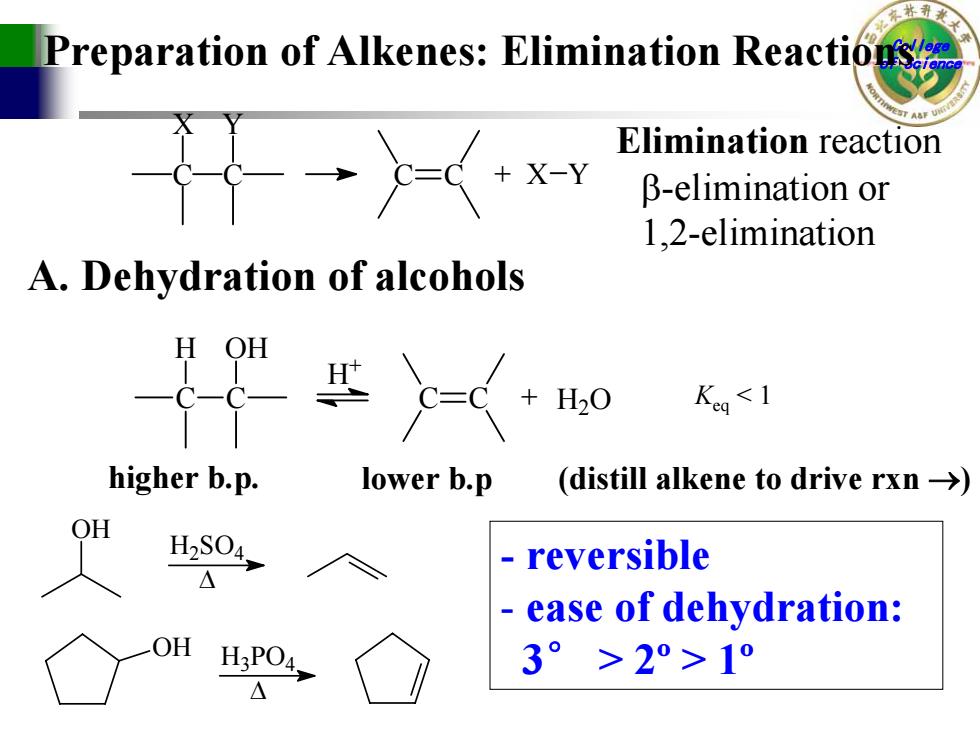
Preparation of Alkenes:Elimination Reactions Elimination reaction X-Y B-elimination or 1.2-elimination A.Dehydration of alcohols H OH H20 Keg H2S04, reversible ease of dehydration: H3PO4 3°>2°>1°
College Preparation of Alkenes: Elimination Reactionsof Science C X C Y C C + X Y Elimination reaction β-elimination or 1,2-elimination A. Dehydration of alcohols C H C OH C C + H 2 O H + Keq 2º > 1º
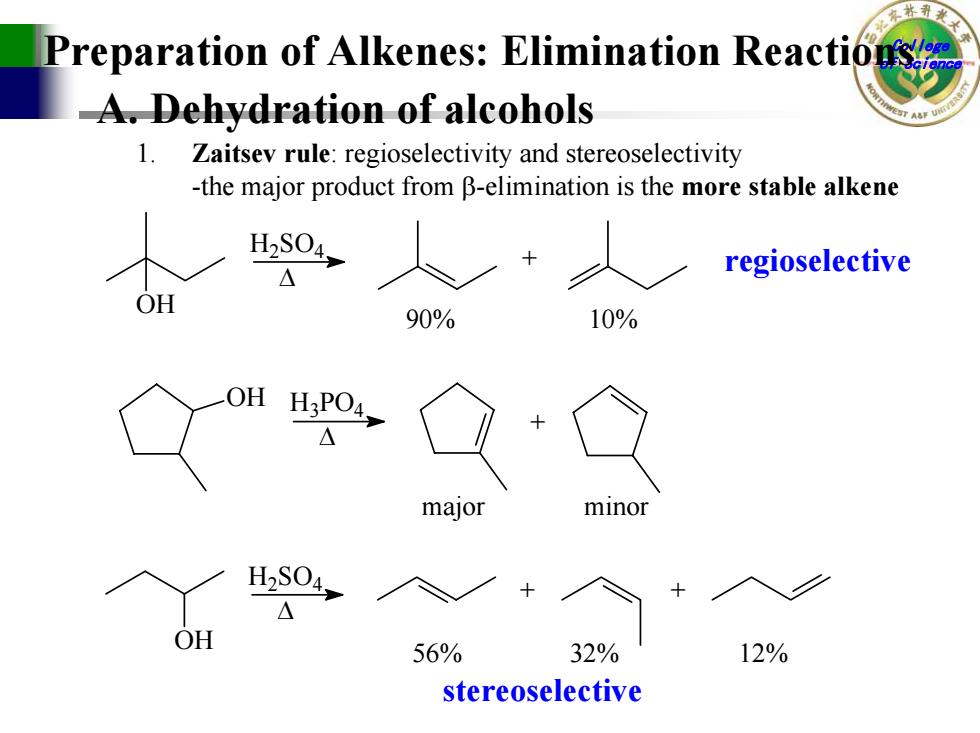
Preparation of Alkenes:Elimination Reaction A.Dehydration of alcohols 1.Zaitsev rule:regioselectivity and stereoselectivity -the major product from B-elimination is the more stable alkene 人 s0人 regioselective O 90% 10% OH HaPO4 major minor H2S04, △ OH 56% 32% 12% stereoselective
College of Science A. Dehydration of alcohols Preparation of Alkenes: Elimination Reactions 1. Zaitsev rule: regioselectivity and stereoselectivity -the major product from β-elimination is the more stable alken e OH + H 2SO 4 ∆ 90% 10% OH H 3PO 4 ∆ + OH + + H 2SO 4 ∆ 56% 32% 12% major minor regioselective stereoselective
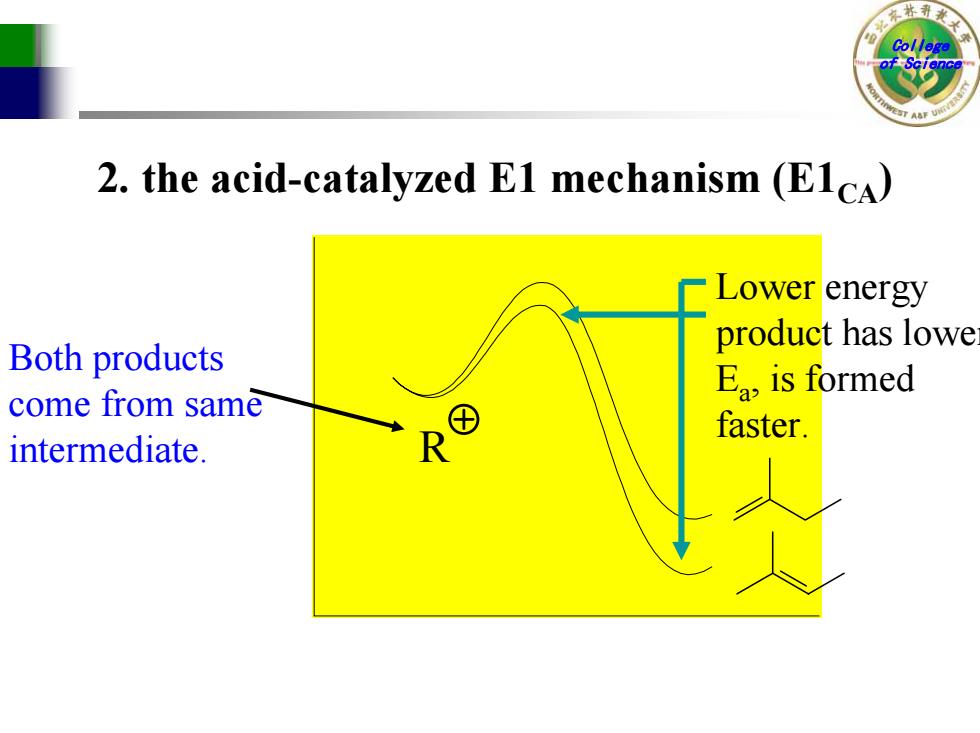
Colle 2.the acid-catalyzed E1 mechanism (EICA) Lower energy product has lowe Both products Ea is formed come from same faster. intermediate
College of Science 2. the acid-catalyzed E1 mechanism (E1CA ) R Both products come from same intermediate. Lower energy product has lowe r E a, is formed faster
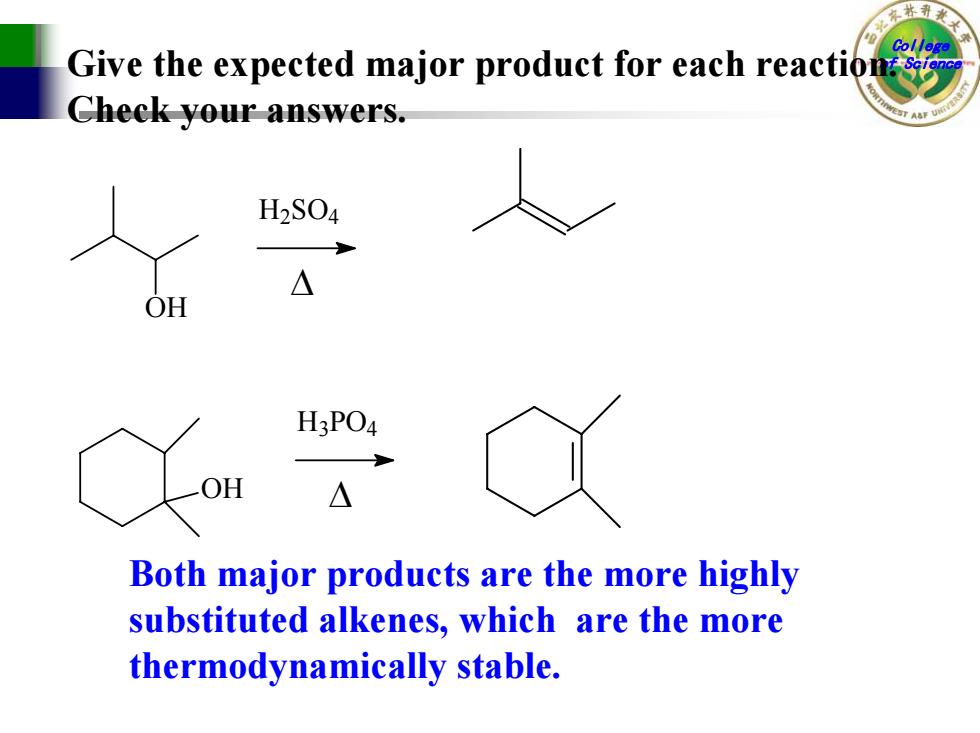
林 Give the expected major product for each reactions Check your answers. H2S04 △ OH H3PO4 OH △ Both major products are the more highly substituted alkenes,which are the more thermodynamically stable
College Give the expected major product for each reaction.of Science Check your answers. OH H 2SO 4 ∆ OH H 3PO 4 ∆ Both major products are the more highly substituted alkenes, which are the more thermodynamically stable
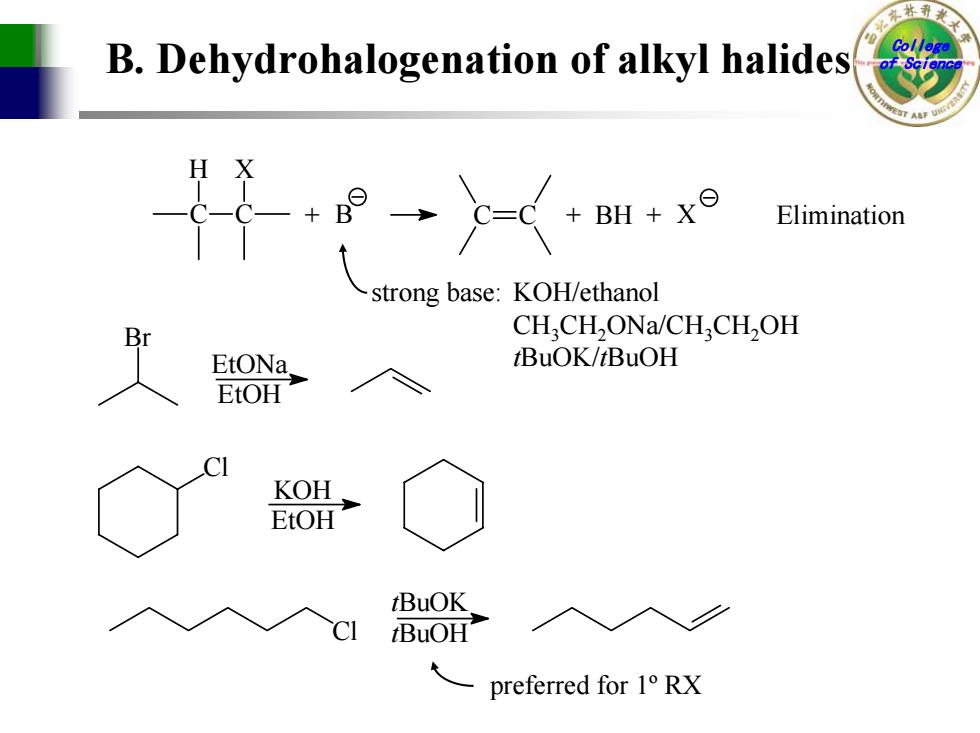
:Q B.Dehydrohalogenation of alkyl halides Colle BH+x Elimination strong base:KOH/ethanol 8, CHCH,ONa/CH,CH,OH EtONa, tBuOK/tBuOH EtOH KOH EtOH tBuOK. BuOH preferred for 1 RX
College B. Dehydrohalogenation of alkyl halides of Science C H C X + B C C + BH + X strong base: K O H/ethanol CH 3CH 2ONa/CH 3CH 2OH tBuOK/ tBuOH Br E tONa Et O H Cl KO H Et O H Cl tBu O K tBu O H Elimination preferred for 1º RX