Conjugated Dienes and U.V.Spectroscopy
Conjugated Dienes and U.V. Spectroscopy
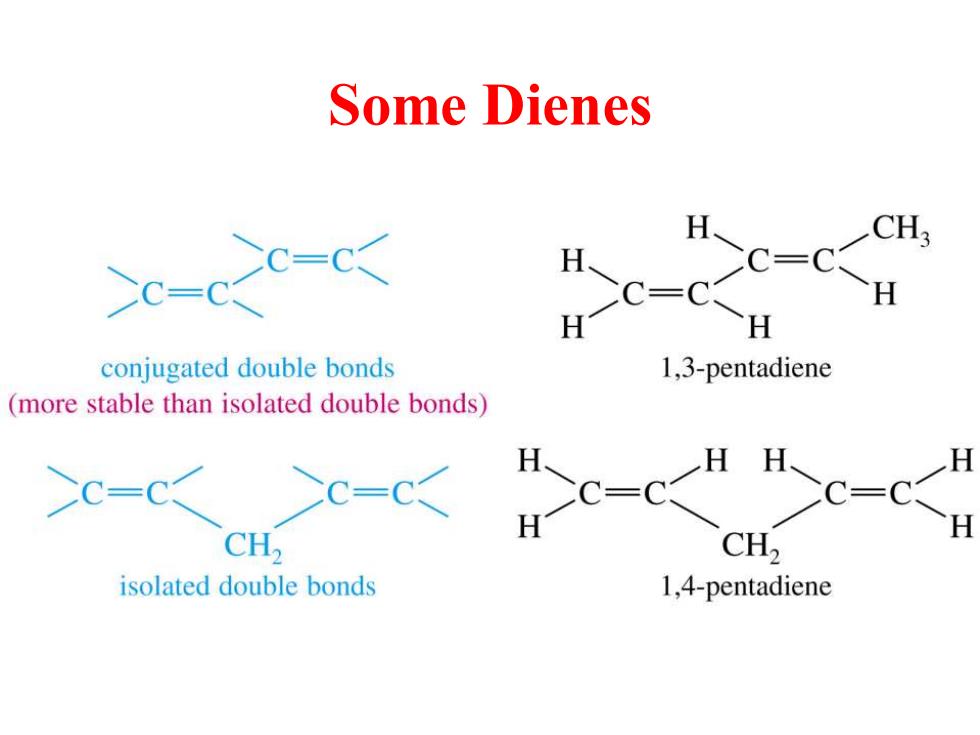
Some Dienes H CH >c-cxc-c< AC兰CU conjugated double bonds L,3-pentadiene (more stable than isolated double bonds) CH, CH, isolated double bonds 1,4-pentadiene
Some Dienes
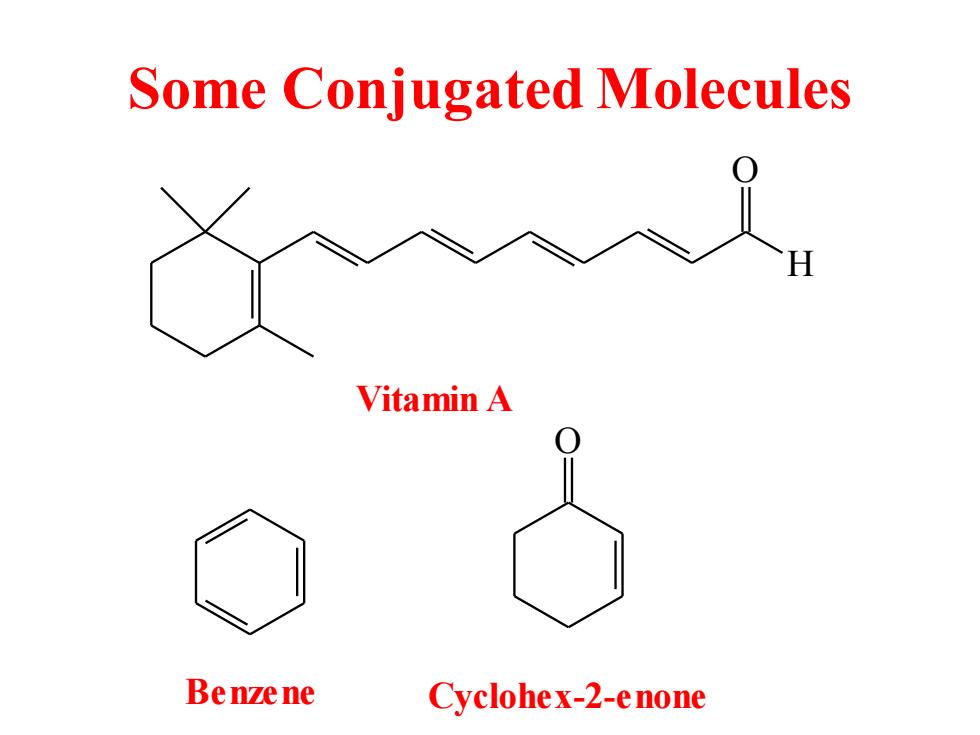
Some Conjugated Molecules Vitamin A Benzene Cyclohex-2-enone
Some Conjugated Molecules O H Vitamin A Benzene O Cyclohex-2-enone
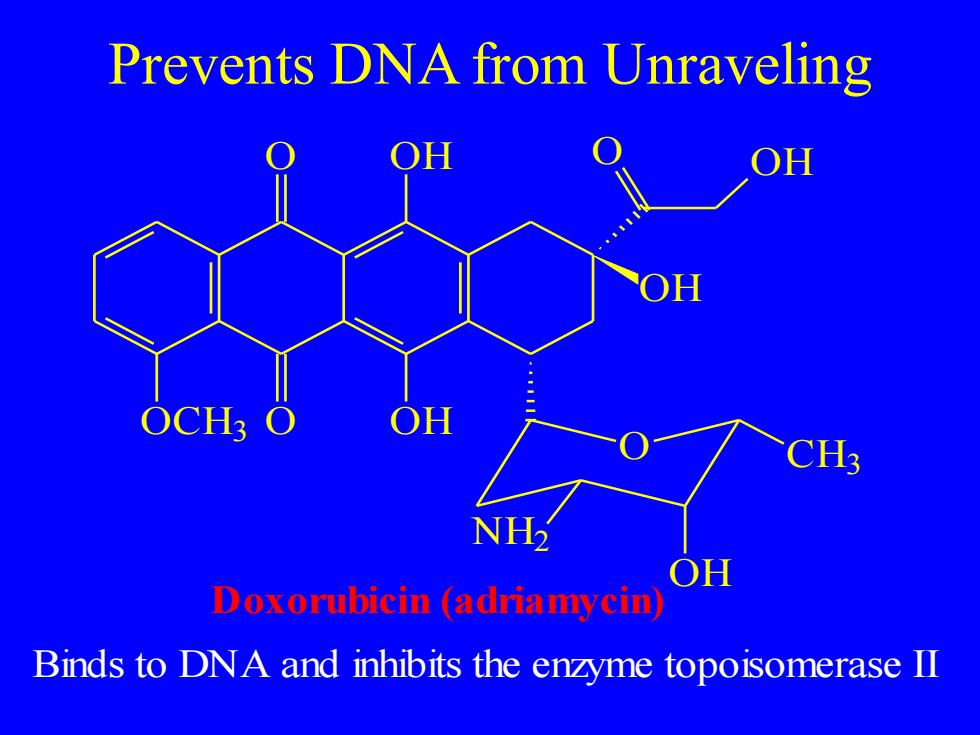
Prevents DNA from Unraveling OH OH OCH3 CH3 NH OH Doxorubicin (adriamycin Binds to DNA and inhibits the enzyme topoisomerase II
Prevents DNA from Unraveling O O OH OH OCH3 O OH OH O NH2 OH CH3 Doxorubicin (adriamycin) Binds to DNA and inhibits the enzyme topoisomerase II
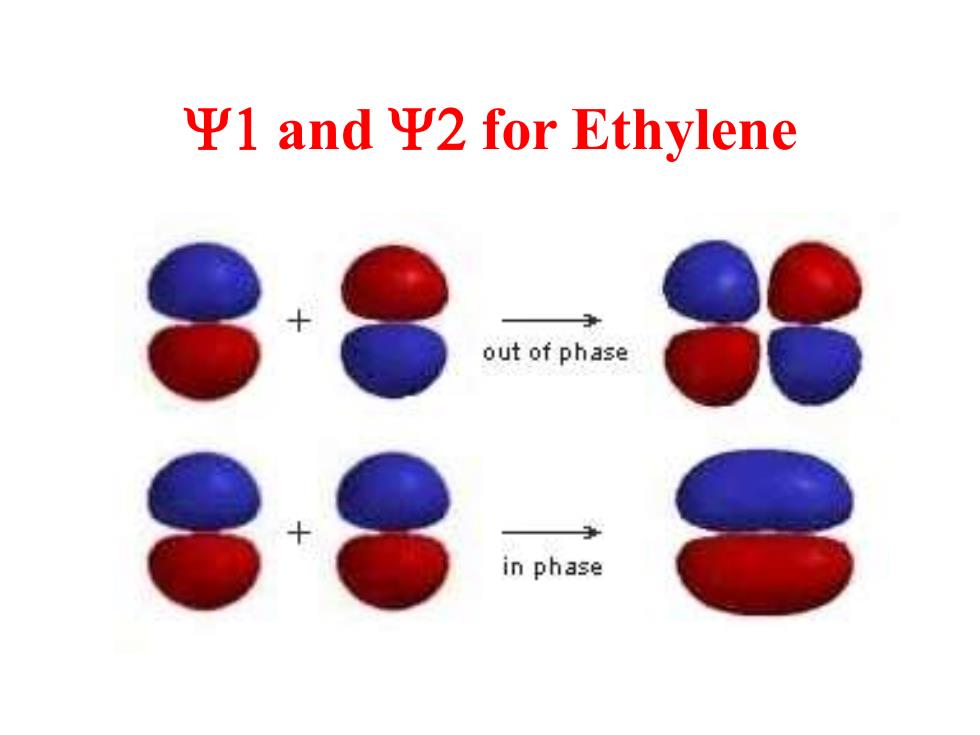
平1andΨ2 for Ethylene out of phase in phase
Y1 and Y2 for Ethylene
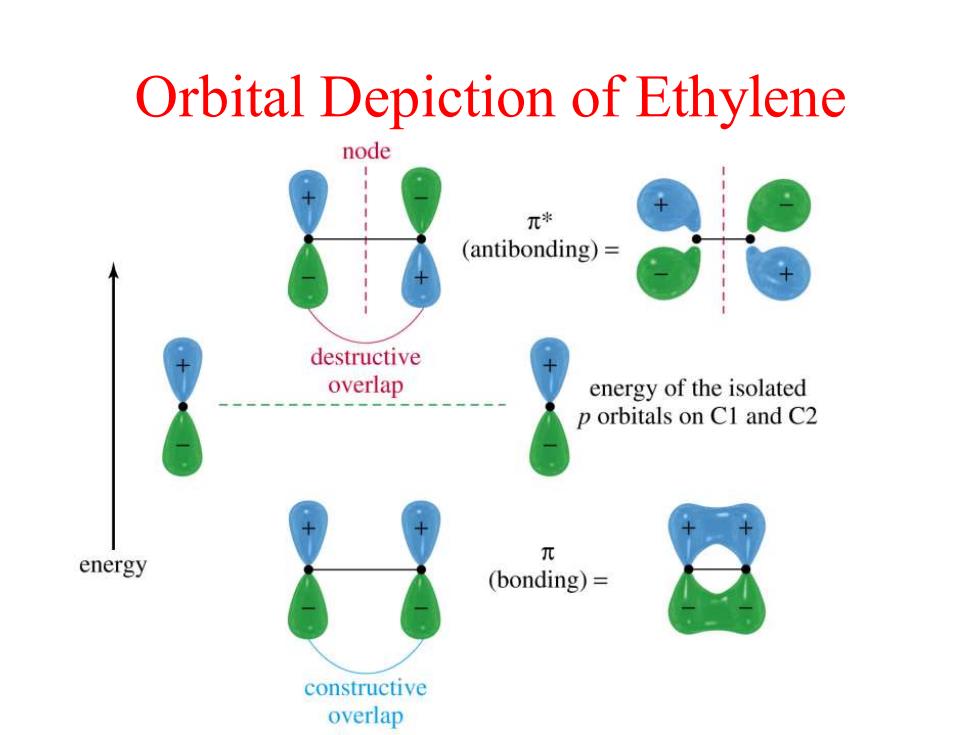
Orbital Depiction of Ethylene node π* (antibonding)= destructive overlap energy of the isolated p orbitals on C1 and C2 energy 元 (bonding)= constructive overlap
Orbital Depiction of Ethylene

Orbital Depiction small amount of overlap partial double bond H 1.34A H H H C 4H 1.48A 1.34A H H
Orbital Depiction
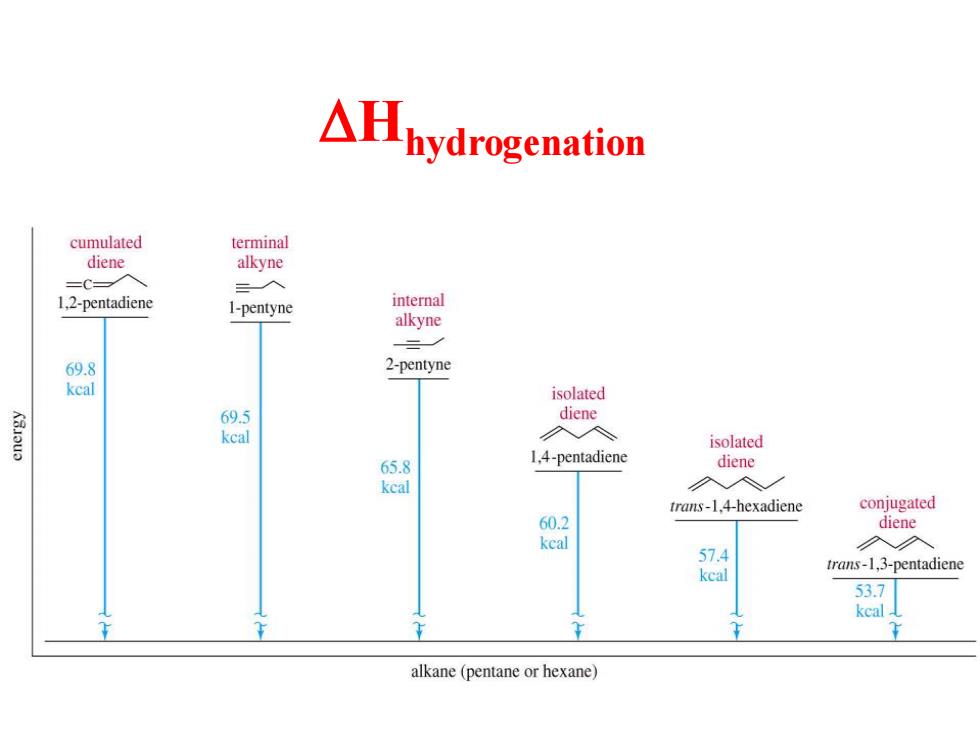
△hydrogenation cumulated terminal diene alkyne =C 1,2-pentadiene 1-pentyne internal alkyne 69.8 2-pentyne kcal isolated K3ou 69.5 diene kcal isolated 65.8 1,4-pentadiene diene kcal trans-1.4-hexadiene conjugated 60.2 diene keal 入◇ 57.4 kcal trans-1,3-pentadiene 53.7 kcal alkane (pentane or hexane)
DHhydrogenation
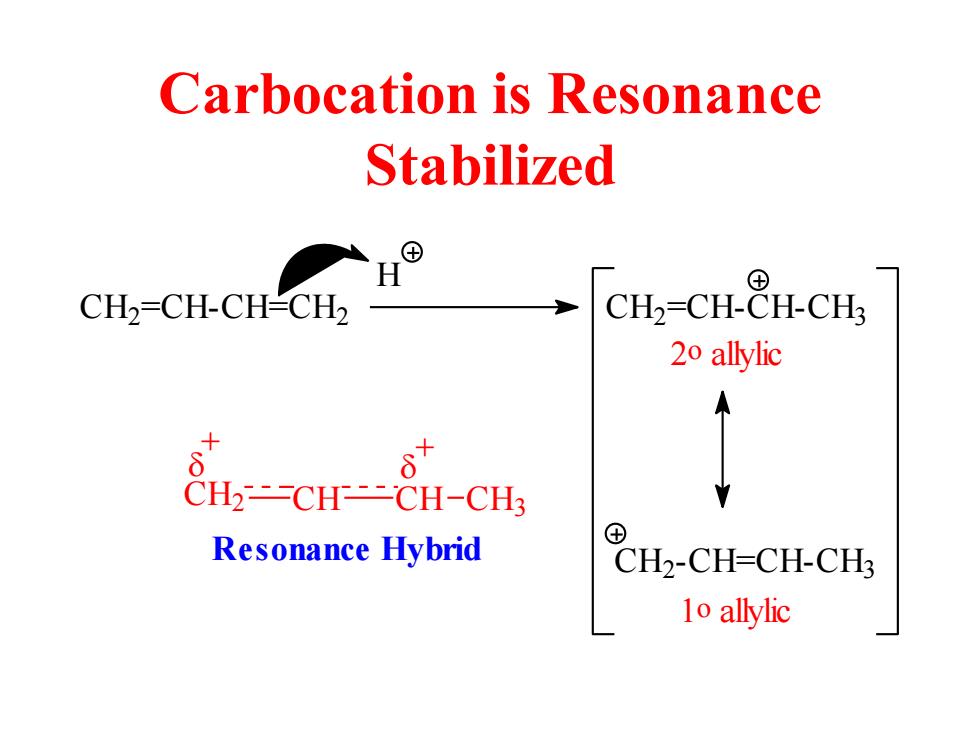
Carbocation is Resonance Stabilized ⊕ H ⊕ CH2=CH-CH-CH2 CH2-CH-CH-CH3 20 allylic CH2-CH-CH-CH3 Resonance Hybrid CH2-CH=CH-CH3 1o allylic
Carbocation is Resonance Stabilized C H2 =CH-CH=CH2 H C H2 =CH-CH-CH3 2o allylic C H2-CH=CH-CH3 1o allylic C H2 CH CH CH3 + + Resonance Hybrid
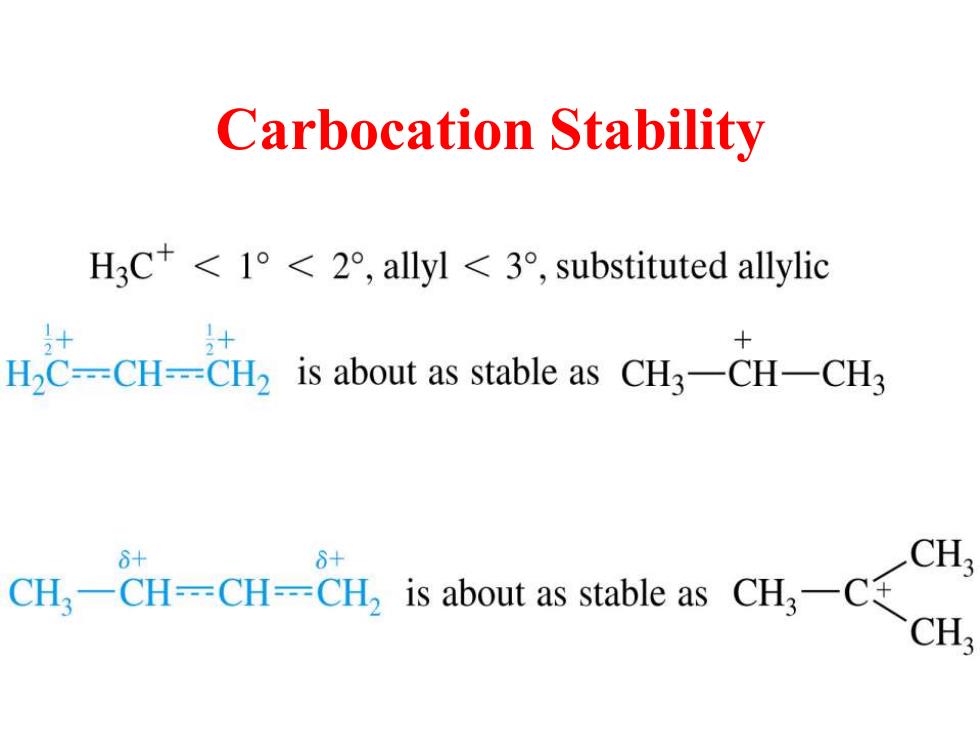
Carbocation Stability H3C+<1°<2°,allyl<3°,substituted allylic H2C--=CH---CH2 is about as stable as CH3-CH-CH3 8+ 6+ CH CH3-CH--CH--CH2 is about as stable as CH3-C+ CH
Carbocation Stability