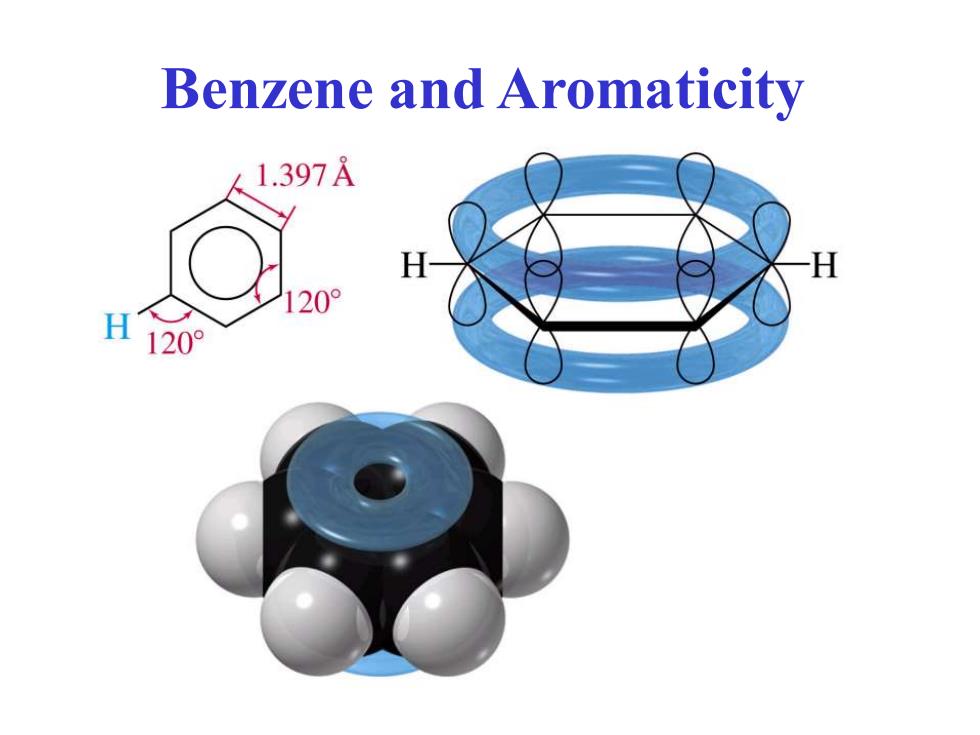
Benzene and Aromaticity 1.397A H H 20° H 120°
Benzene and Aromaticity
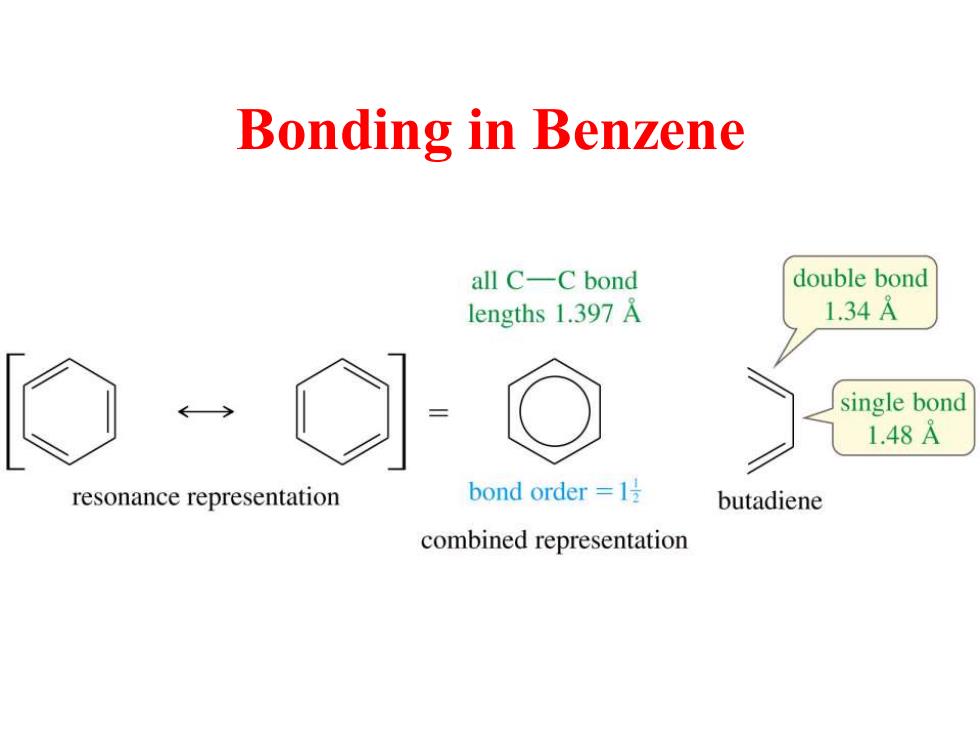
Bonding in Benzene all C-C bond double bond lengths 1.397 A 1.34A single bond 1.48A resonance representation bond order =1 butadiene combined representation
Bonding in Benzene
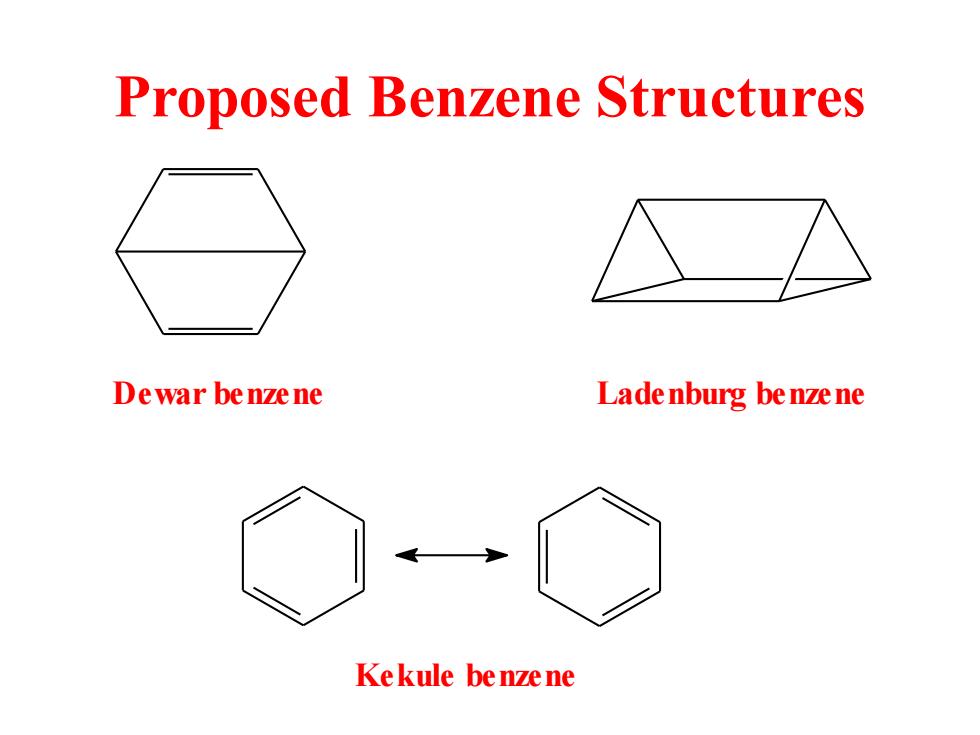
Proposed Benzene Structures Dewar benzene Lade nburg be nze ne Kekule be nze ne
Proposed Benzene Structures Dewar benzene Ladenburg benzene Kekule benzene
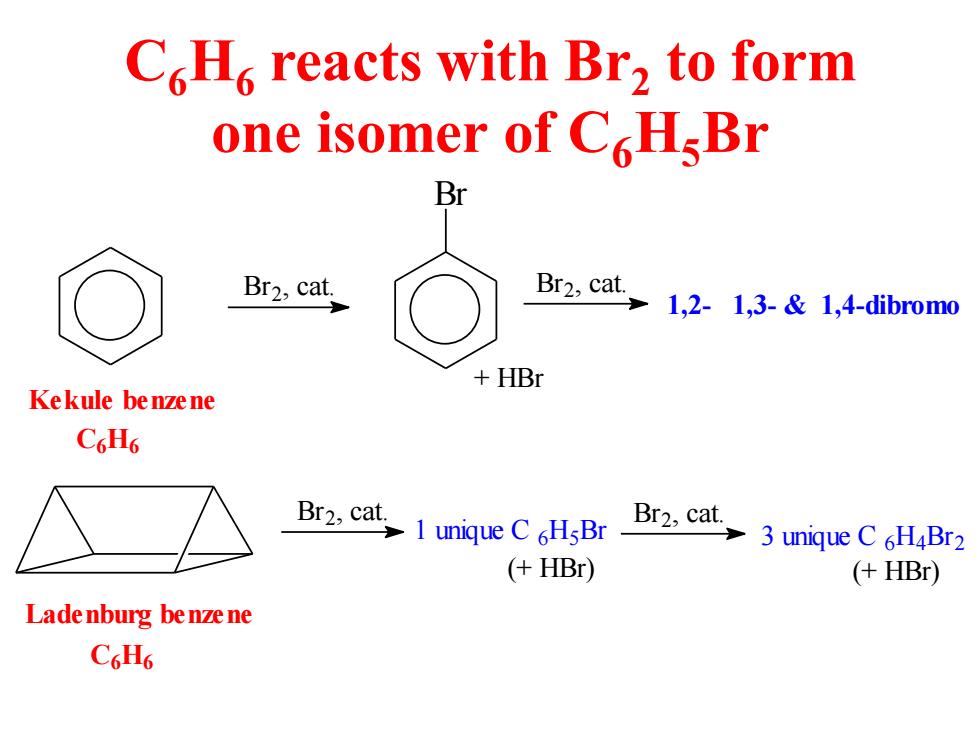
C.H reacts with Br2 to form one isomer of CHsBr Br Br2,cat. Br2,cat 12-1,3-&1,4-dibromo +HBr Kekule benzene C6H6 Br2.caluqueCHBr Br cat 3 unique C 6H4Br2 (+HBr) (+HBr) Lade nburg be nze ne C6H6
C6H6 reacts with Br2 to form one isomer of C6H5Br Ladenburg benzene Kekule benzene Br2 , cat. 1 unique C 6H5Br Br2, cat. 3 unique C 6H4Br2 (+ HBr) (+ HBr) C6H6 C6H6 Br2, cat. Br Br2, cat. 1,2- 1,3- & 1,4-dibromo + HBr
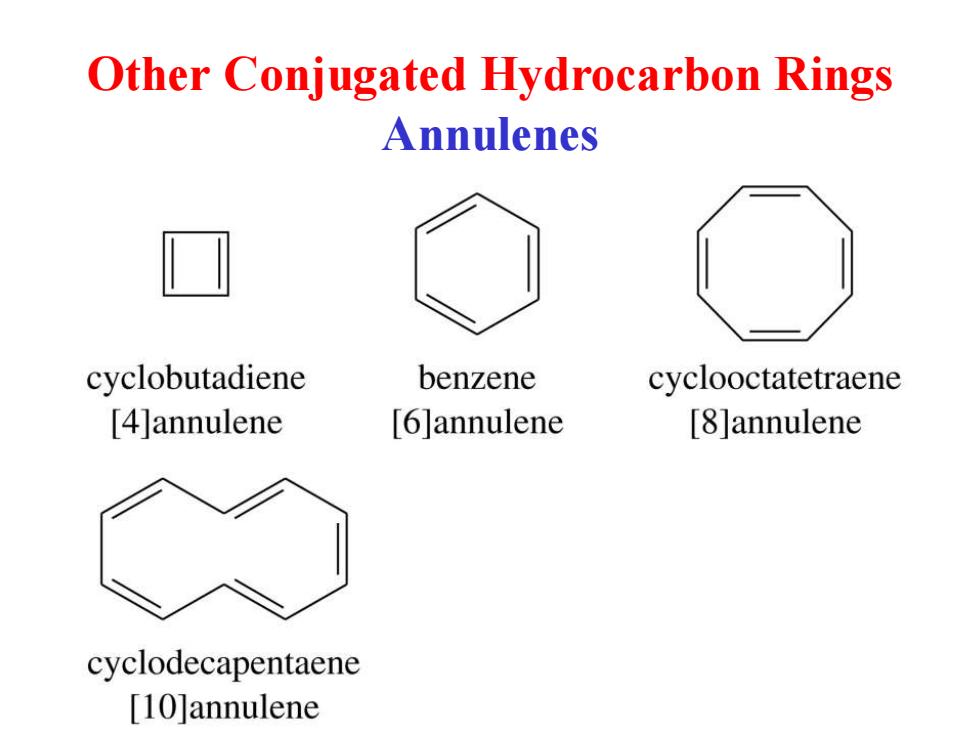
Other Conjugated Hydrocarbon Rings Annulenes cyclobutadiene benzene cyclooctatetraene [4]annulene [6]annulene [8]annulene cyclodecapentaene [10]annulene
Other Conjugated Hydrocarbon Rings Annulenes
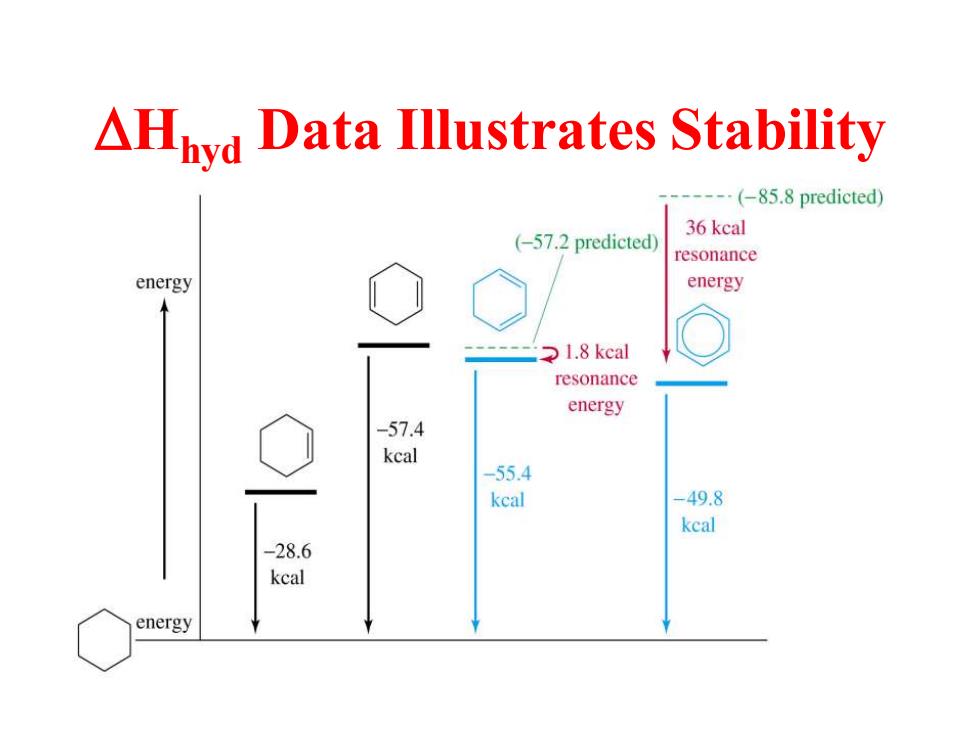
AHyd Data Illustrates Stability ----(-85.8 predicted) (-57.2 predicted) 36 kcal resonance energy energy 1.8 keal resonance energy -57.4 kcal -55.4 keal -49.8 keal -28.6 keal energy
DHhyd Data Illustrates Stability
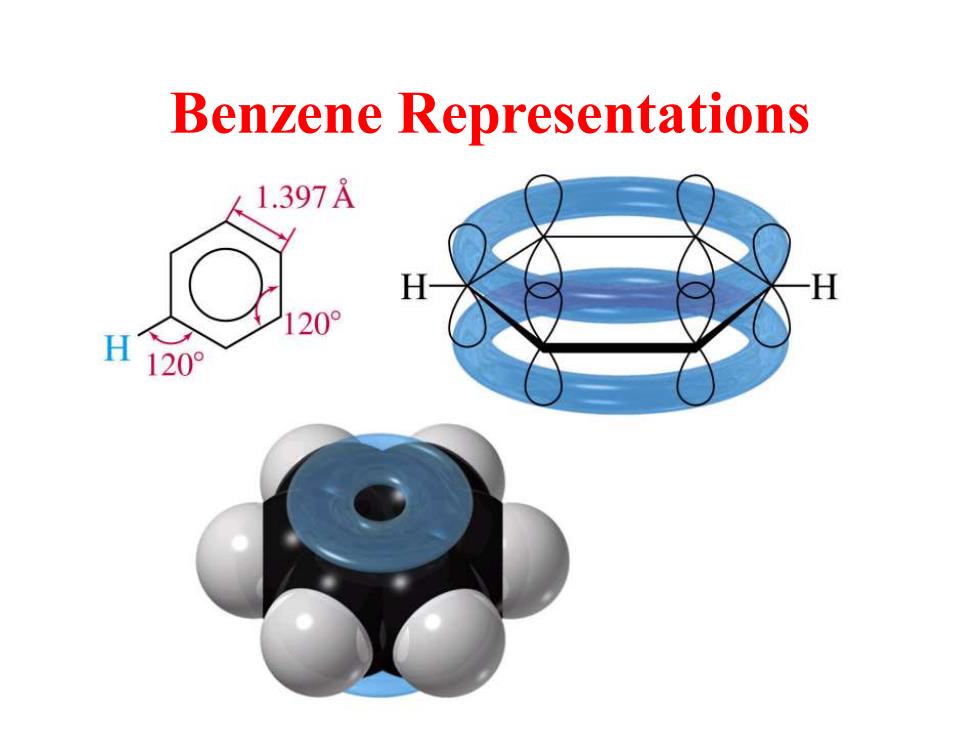
Benzene Representations 1.397A 120° H 120°
Benzene Representations
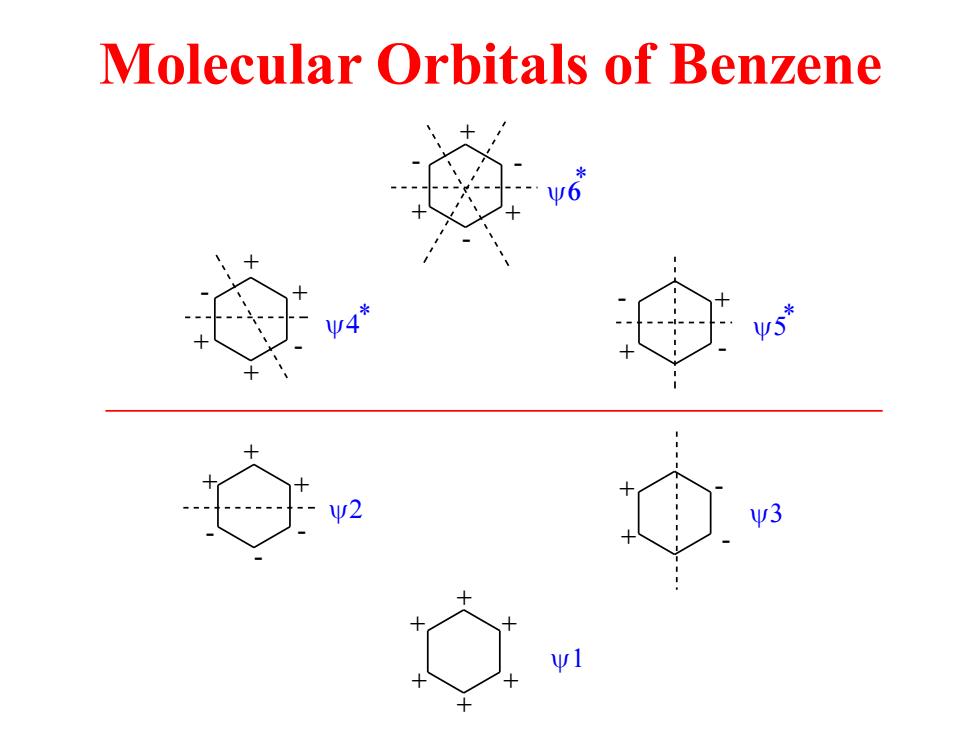
Molecular Orbitals of Benzene 6 y4* y3
Molecular Orbitals of Benzene + + + + + + + + + + + + + + + + + + + - - - - - + - - - - - - - * * *
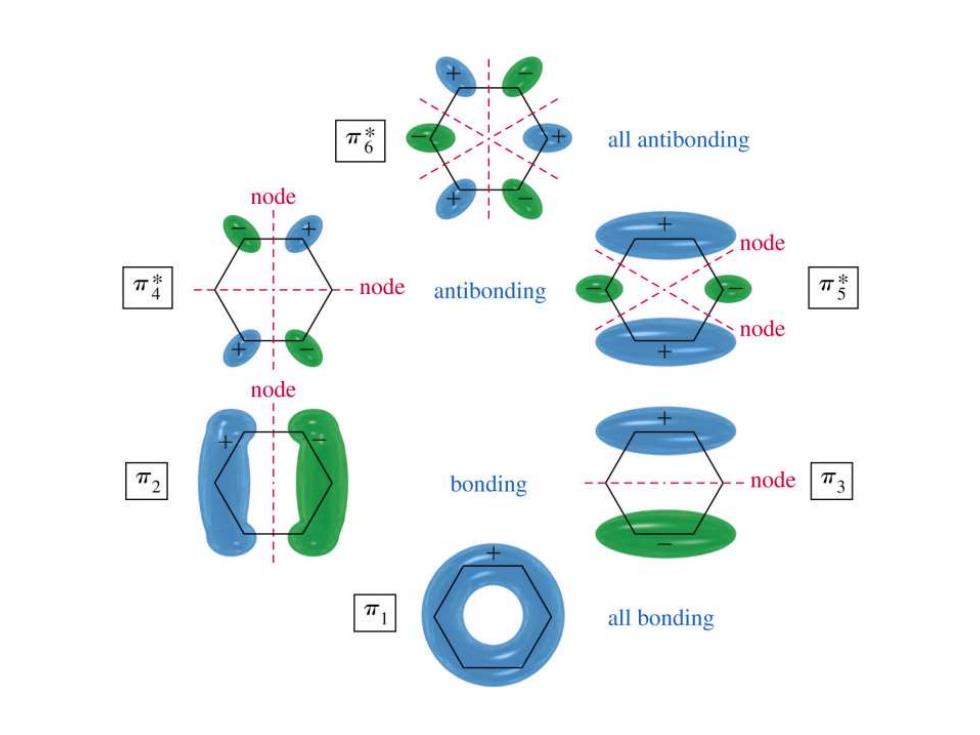
all antibonding node node node antibonding π考 node node T2 bonding node 不3 all bonding
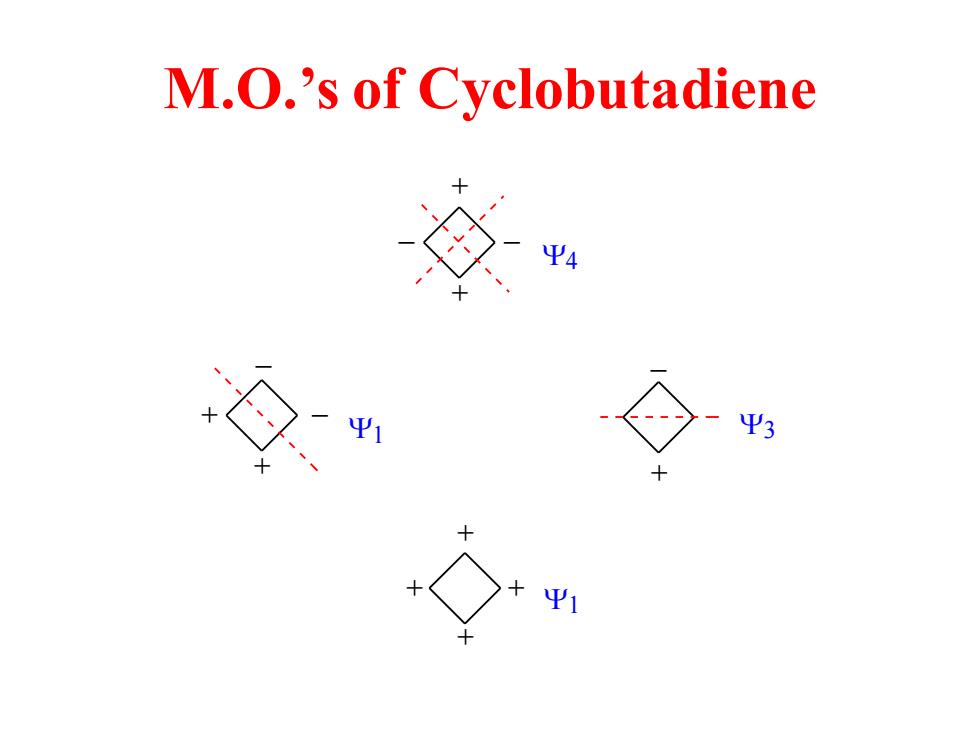
M.O.'s of Cyclobutadiene Ψ3
M.O.’s of Cyclobutadiene + + + + + + _ _ + _ + + _ _ 1 3 1 4