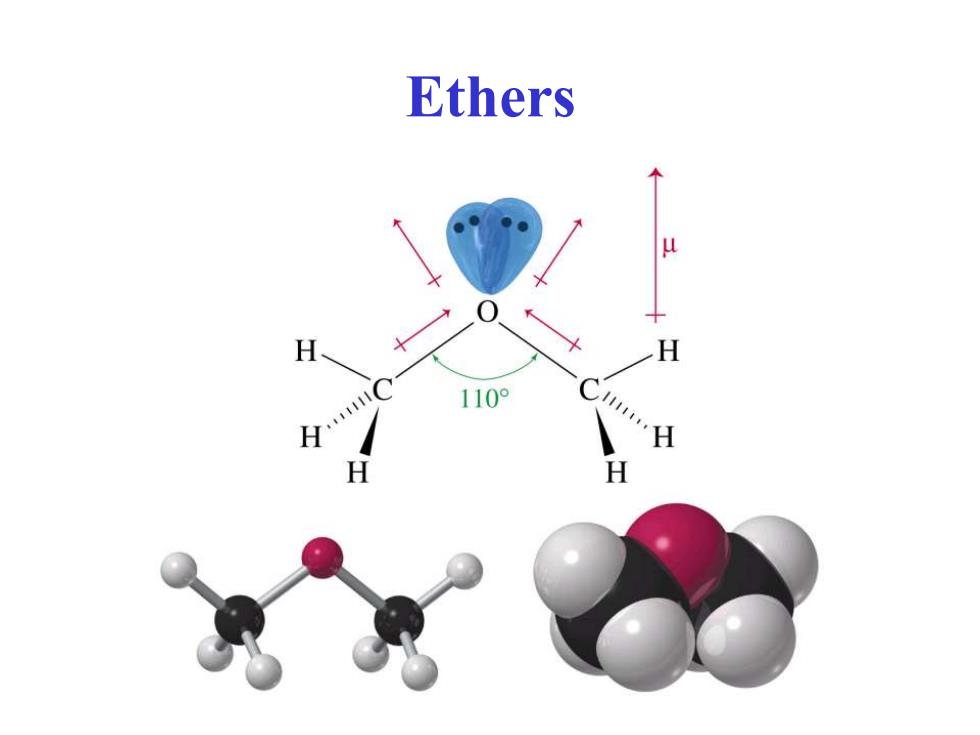
Ethers H H
Ethers
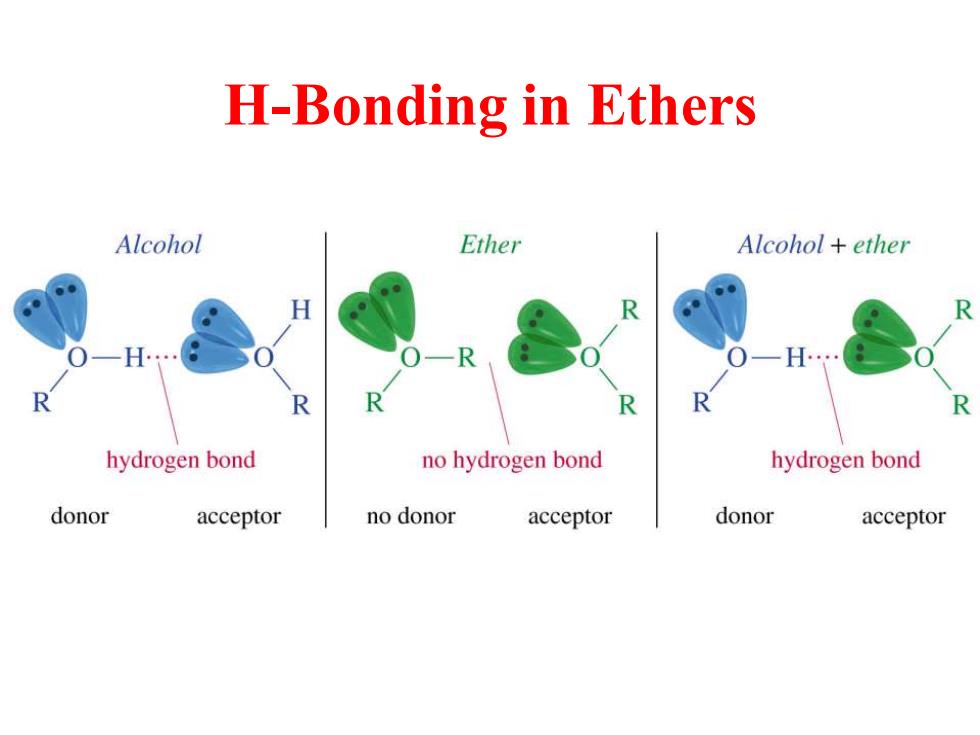
H-Bonding in Ethers Alcohol Ether Alcohol ether H R O-R R R R R hydrogen bond no hydrogen bond hydrogen bond donor acceptor no donor acceptor donor acceptor
H-Bonding in Ethers
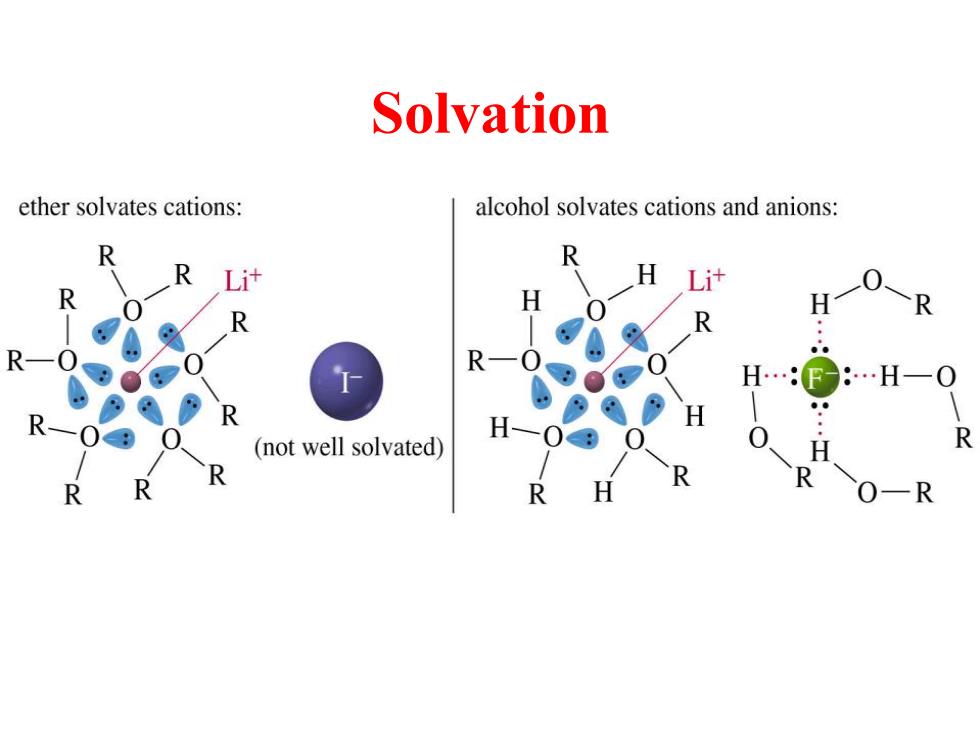
Solvation ether solvates cations: alcohol solvates cations and anions: R R R R H… …H (not well solvated) R R O-R
Solvation
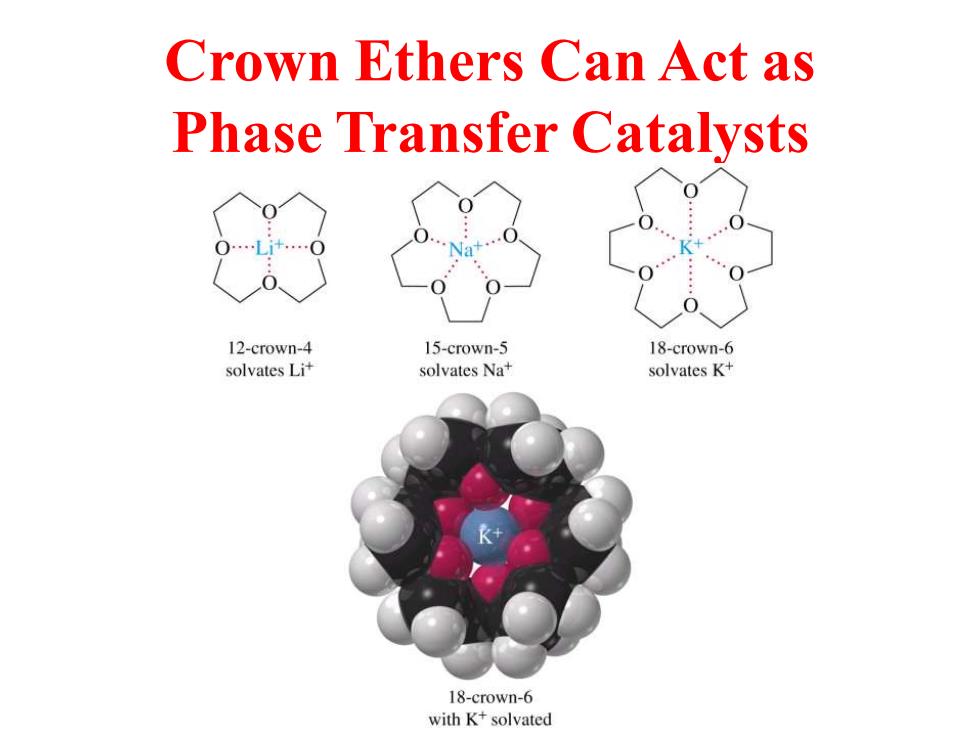
Crown Ethers Can Act as Phase Transfer Catalysts 12-crown-4 15-crown-5 18-crown-6 solvates Lit solvates Na+ solvates K+ 18-crown-6 with K+solvated
Crown Ethers Can Act as Phase Transfer Catalysts
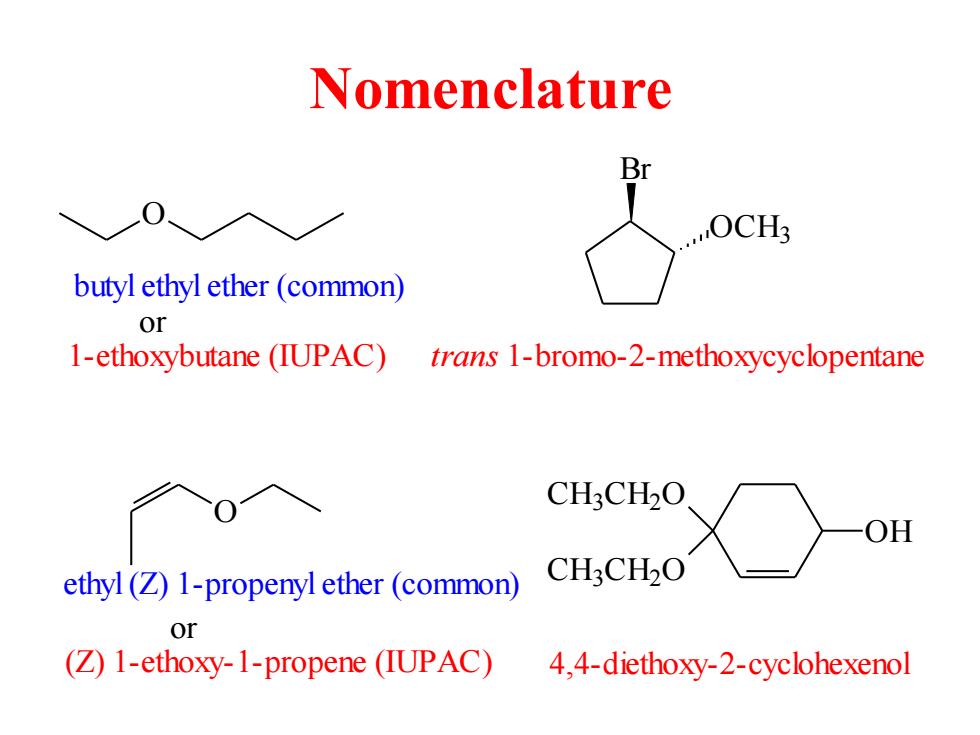
Nomenclature butyl ethyl ether(common) or 1-ethoxybutane (IUPAC) trans 1-bromo-2-methoxycyclopentane CH3CH2O OH ethyl(Z)1-propenyl ether(common) CH:CH2O or (Z)1-ethoxy-1-propene (IUPAC) 4,4-diethoxy-2-cyclohexenol
Nomenclature O butyl ethyl ether (common) 1-ethoxybutane (IUPAC) or OCH3 Br trans 1-bromo-2-methoxycyclopentane ethyl (Z) 1-propenyl ether (common) (Z) 1-ethoxy-1-propene (IUPAC) or OH CH3 CH2 O CH3 CH2 O 4,4-diethoxy-2-cyclohexenol O
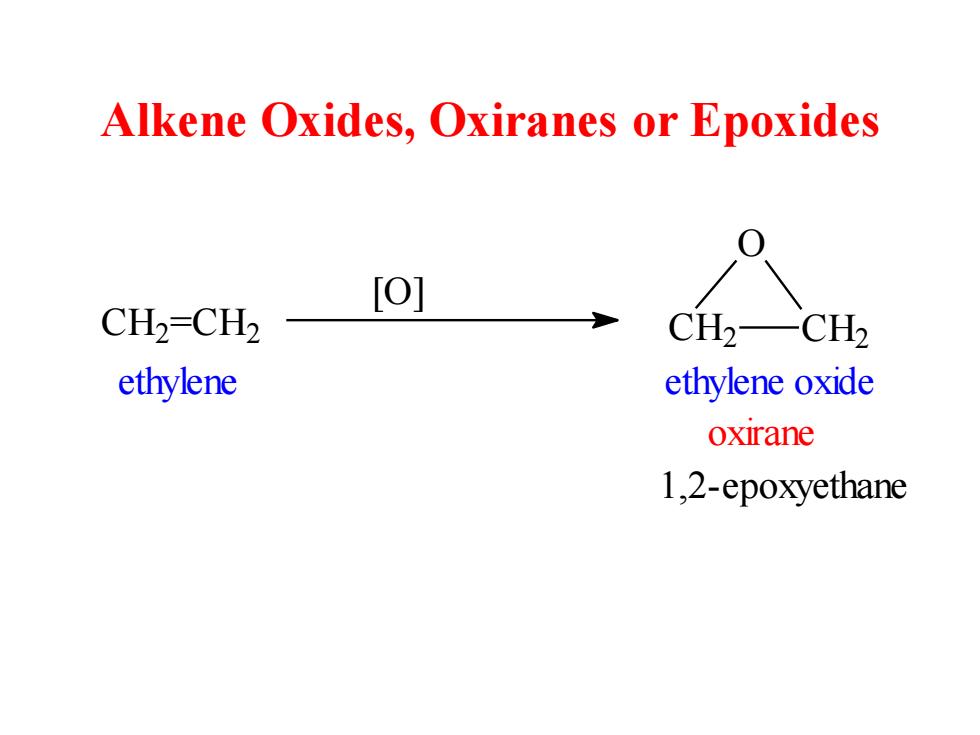
Alkene Oxides,Oxiranes or Epoxides [O] CH=CH2 CH2—CH2 ethylene ethylene oxide oxirane 1,2-epoxyethane
Alkene Oxides, Oxiranes or Epoxides C H2 =CH2 [O] C H2 CH2 O ethylene ethylene oxide oxirane 1,2-epoxyethane
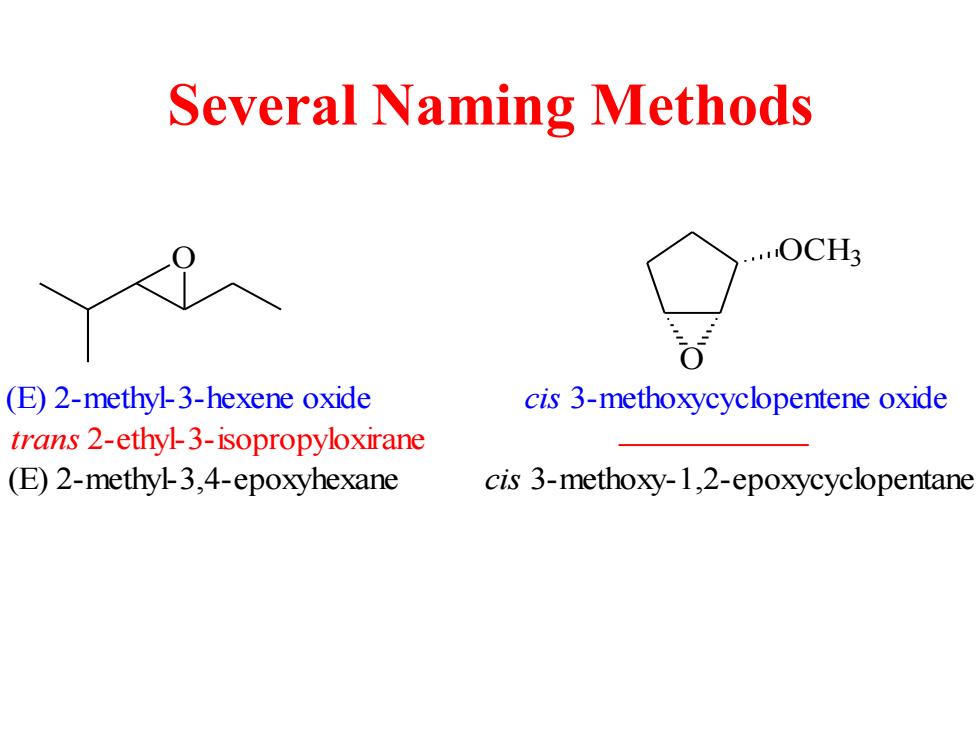
Several Naming Methods ....OCH3 (E)2-methyl-3-hexene oxide cis 3-methoxycyclopentene oxide trans 2-ethyl-3-isopropyloxirane (E)2-methyl-3,4-epoxyhexane cis 3-methoxy-1,2-epoxycyclopentane
Several Naming Methods O (E) 2-methyl-3,4-epoxyhexane (E) 2-methyl-3-hexene oxide trans 2-ethyl-3-isopropyloxirane O OCH3 cis 3-methoxycyclopentene oxide cis 3-methoxy-1,2-epoxycyclopentane
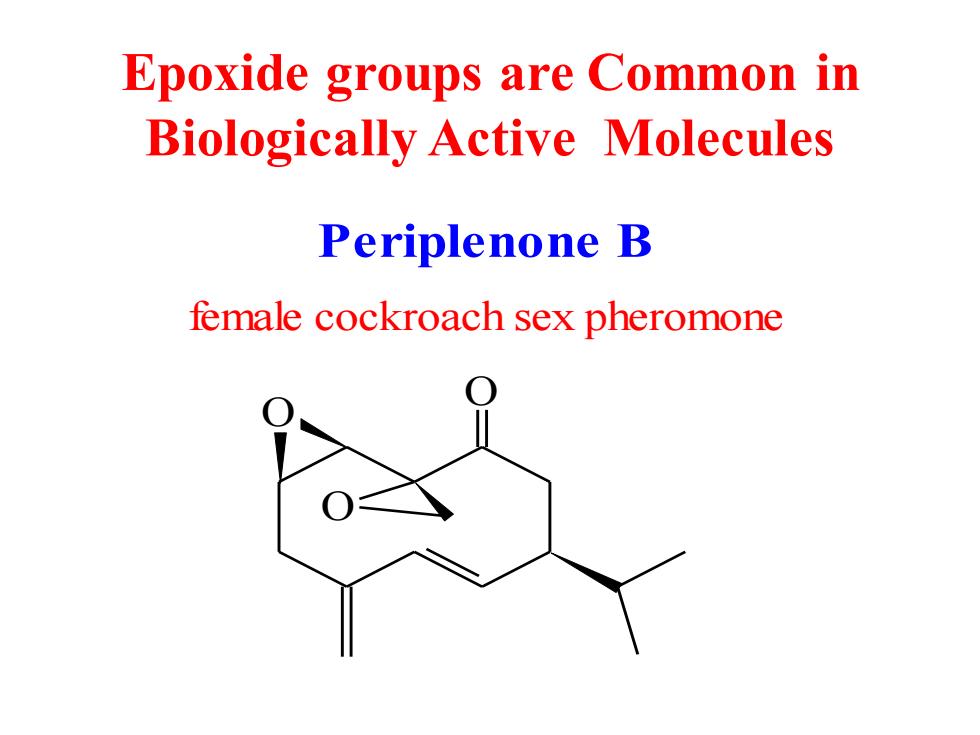
Epoxide groups are Common in Biologically Active Molecules Periplenone B female cockroach sex pheromone
Epoxide groups are Common in Biologically Active Molecules Periplenone B O O O female cockroach sex pheromone
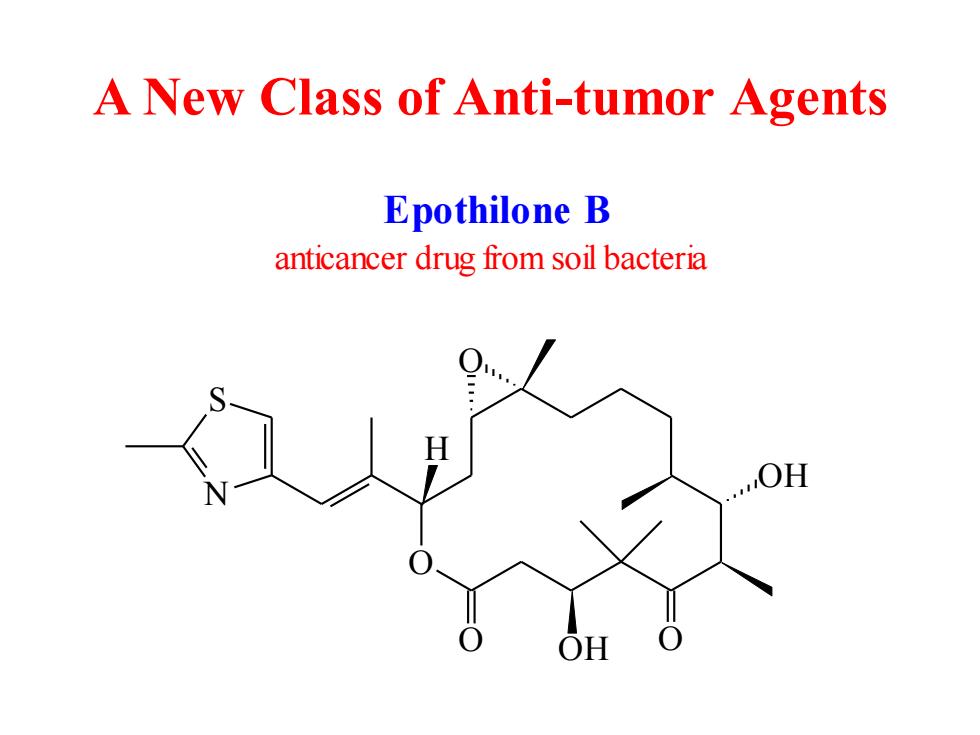
A New Class of Anti-tumor Agents Epothilone B anticancer drug from soil bacteria OH
A New Class of Anti-tumor Agents O OH O OH O O S N H Epothilone B anticancer drug from soil bacteria
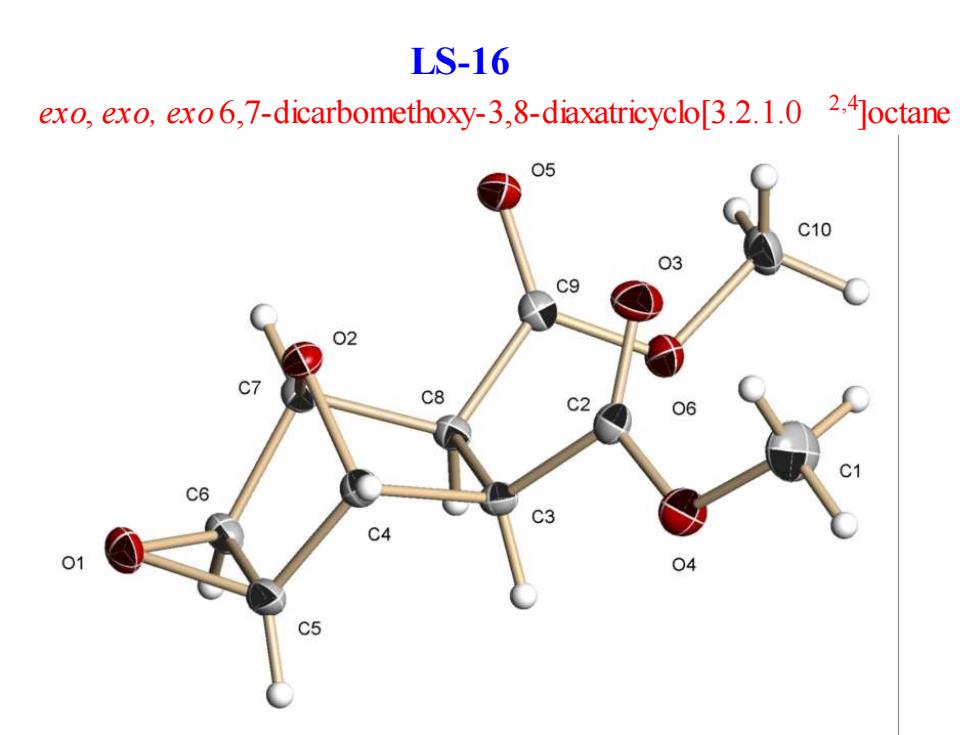
LS-16 exo,exo,exo6,7-dicarbomethoxy-3,8-diaxatricyclo[3.2.1.0 2.4Joctane 05 C10 03 c9 02 C8 C2 c6 C4 04
exo, exo, exo 6,7-dicarbomethoxy-3,8-diaxatricyclo[3.2.1.0 2,4]octane LS-16