自秋不转大对 招单院 Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 3 Brief Introduction and Nomenclature of OCs Key Notes Functionalgroup;Classification;IVPACNames By Junru Wang Email:wangjr07@163.com
By Junru Wang Email: wangjr07@163.com Chapter 3 Brief Introduction and Nomenclature of OCs Organic Chemistry, 6th Edition L. G. Wade, Jr. Key Notes Functional group; Classification; IUPAC Names
自秋标转材 CONTENT Classification Functional groups of O.Cs. Nomenclature of hydrocarbons Alkanes,Alkenes,Alkynes Aromatic hydrocarbons Nomenclature of O.Cs.containing oxygen ●Alcohols,Ehters Aldehydes and ketones Carboxylic acids,Carboxylic acid derivatives Nomenclature of O.Cs.containing nitrogen .Amines,Nitriles,Acid anhlides acid anhydrides Nomenclature of Multifunctional compounds
CONTENT ◼Classification & Functional groups of O.Cs. ◼Nomenclature of hydrocarbons ⚫Alkanes,Alkenes,Alkynes ⚫Aromatic hydrocarbons ◼Nomenclature of O.Cs. containing oxygen ⚫Alcohols,Ehters ⚫Aldehydes and ketones ⚫Carboxylic acids,Carboxylic acid derivatives ◼Nomenclature of O.Cs. containing nitrogen ⚫Amines,Nitriles,Acid anhlides & acid anhydrides ◼Nomenclature of Multifunctional compounds

SEC 1 Classes of Compounds Classification based on functional group. ■Three broad classes ●Hydrocarbons Compounds containing oxygen .Compounds containing nitrogen
SEC 1 Classes of Compounds ◼Classification based on functional group. ◼Three broad classes ⚫Hydrocarbons ⚫Compounds containing oxygen ⚫Compounds containing nitrogen
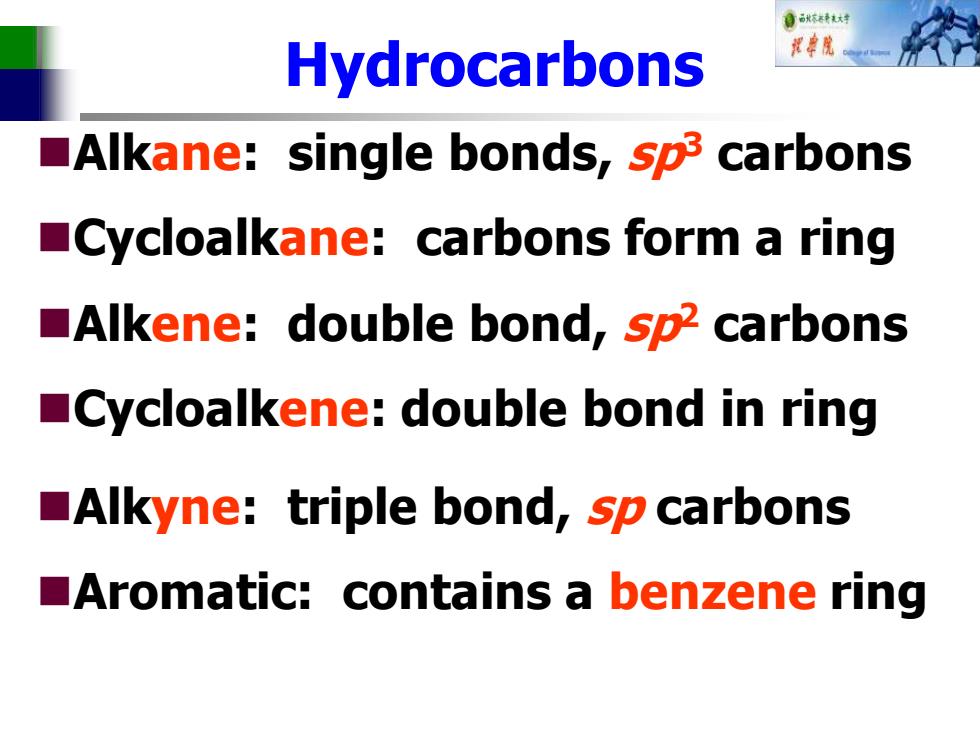
自秋特大材 Hydrocarbons Alkane:single bonds,sp3 carbons Cycloalkane:carbons form a ring Alkene:double bond,sp2 carbons Cycloalkene:double bond in ring Alkyne:triple bond,sp carbons Aromatic:contains a benzene ring
Hydrocarbons ◼Alkane: single bonds, sp3 carbons ◼Cycloalkane: carbons form a ring ◼Alkene: double bond, sp2 carbons ◼Cycloalkene: double bond in ring ◼Alkyne: triple bond, sp carbons ◼Aromatic: contains a benzene ring
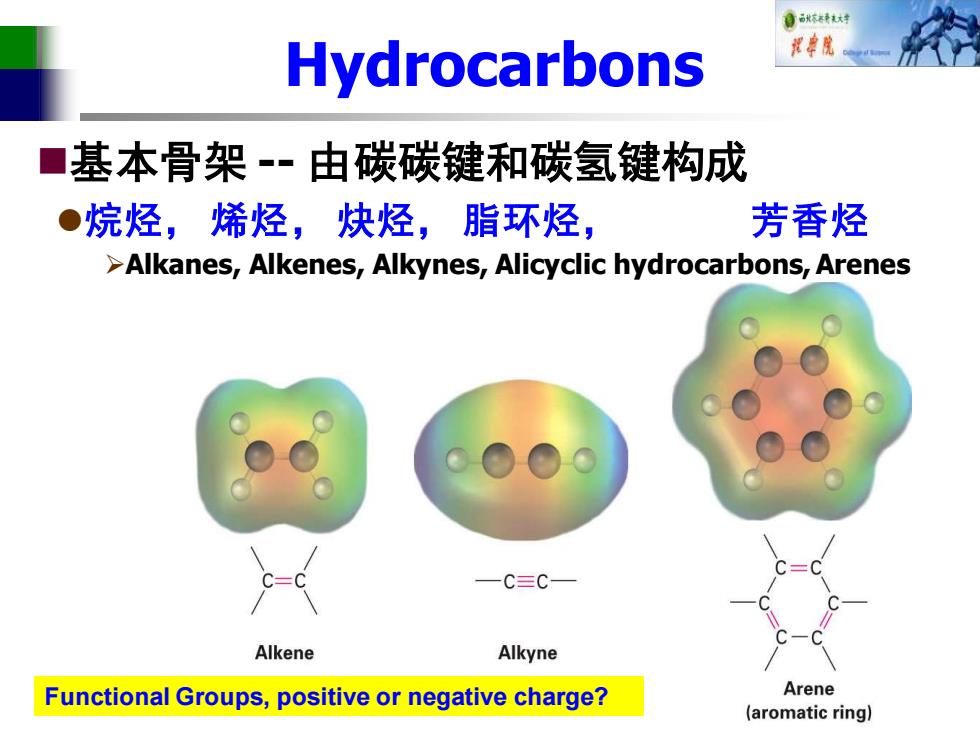
自秋不转大对 Hydrocarbons 视中院 ■ 基本骨架-·由碳碳键和碳氢键构成 ●烷烃,烯烃,炔烃,脂环烃, 芳香烃 >Alkanes,Alkenes,Alkynes,Alicyclic hydrocarbons,Arenes 一C三C一 Alkene Alkyne Functional Groups,positive or negative charge? Arene (aromatic ring)
Hydrocarbons ◼基本骨架 -- 由碳碳键和碳氢键构成 ⚫烷烃, 烯烃, 炔烃, 脂环烃, 芳香烃 ➢Alkanes, Alkenes, Alkynes, Alicyclic hydrocarbons, Arenes Functional Groups, positive or negative charge?
自秋不特大对 Predict how any one of those compounds reacts by analyzing its “functional groups” Functional Group-group of atoms with characteristic chemical behavior no matter what molecule it's in Chemistry of every organic molecule,regardless of size or complexity, governed by functional groups

自秋转达对 Functional Groups ■官能团(Functional Groups): The atom or group ofatoms that defines the structure of a particular family oforganic compounds and,at the same time determines their properties Structuralfeatures that allow for classification of compounds into families The structuralsimilarities in these compounds lead to chemical similarities ●官能团:有机分子中比较活泼、易发生化学反应的 原子或原子团 ●官能团:赋予有机物一类特征结构并决定其性质的 原子或原子团
Functional Groups ◼官能团(Functional Groups): ⚫The atom or group of atoms that defines the structure of a particular family of organic compounds and, at the same time determines their properties ⚫Structural features that allow for classification of compounds into families ⚫The structural similarities in these compounds lead to chemical similarities ⚫官能团: 有机分子中比较活泼、易发生化学反应的 原子或原子团 ⚫官能团: 赋予有机物一类特征结构并决定其性质的 原子或原子团

自秋特大材 Functional Groups It should be noted that the importance of a functional group cannot be overstated. A functional group determines all of the following properties of a molecule: ● Bonding and shape Chemical reactivity ● Type and strength of intermolecular forces Physical properties Nomenclature
Functional Groups ◼It should be noted that the importance of a functional group cannot be overstated. ◼A functional group determines all of the following properties of a molecule: ⚫ Bonding and shape ⚫ Chemical reactivity ⚫ Type and strength of intermolecular forces ⚫ Physical properties ⚫ Nomenclature
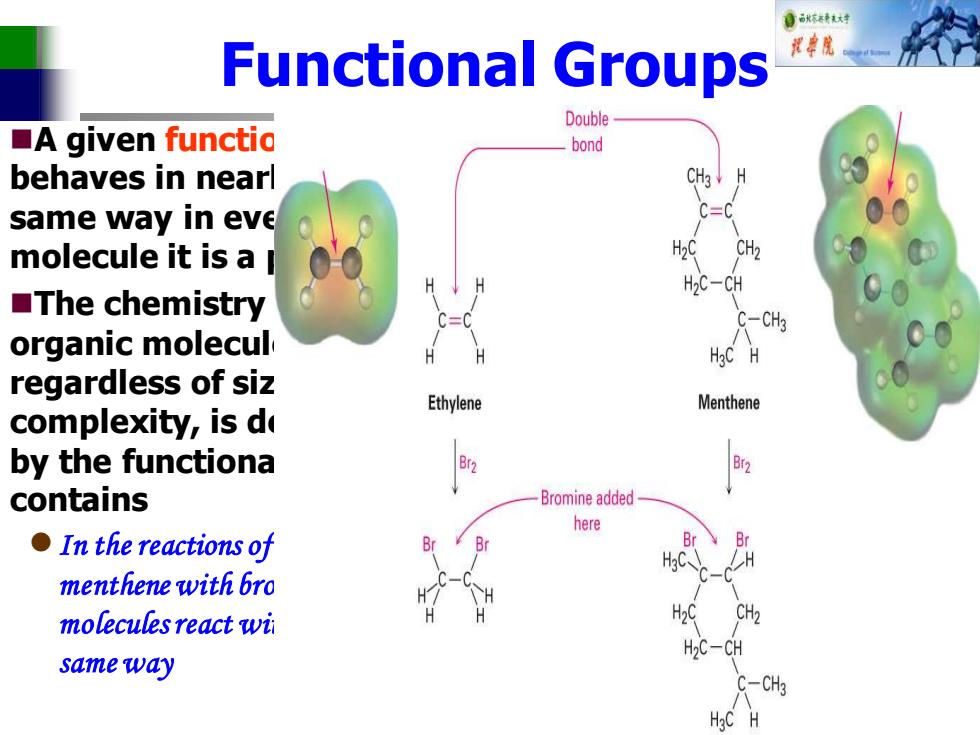
自秋转达对 Functional Groups Double ■A given functio bond behaves in nearl same way in eve molecule it is a H20 CH2 H2C-CH ■The chemistry C-CH3 organic molecul regardless of siz Ethylene Menthene complexity,is de by the functiona contains Bromine added here ●In the reactions of B menthene with bro molecules react wi H2C-CH same way 一CH
Functional Groups ◼A given functional group behaves in nearly the same way in every molecule it is a part of ◼The chemistry of every organic molecule, regardless of size and complexity, is determined by the functional groups it contains ⚫In the reactions of ethylene and menthene with bromine both molecules react with Br2 in the same way
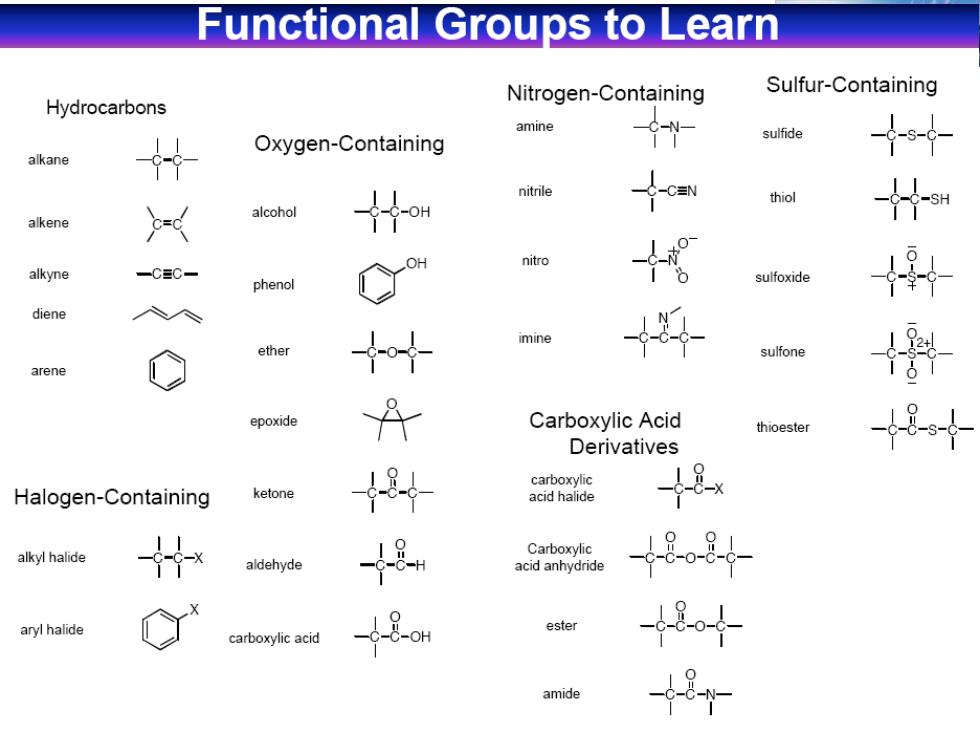
Functional Groups to Learn Hydrocarbons Nitrogen-Containing Sulfur-Containing amine Oxygen-Containing sulfide alkane nitrile thiol alcohol alkene nitro alkyne 一CC一 phenol sulfoxide diene imine ether sulfone arene epoxide Carboxylic Acid thioester Derivatives carboxylic Halogen-Containing ketone acid halide -8x alkyl halide Carboxylic aldehyde acid anhydride 18-08 aryl halide ester carboxylic acid 484 amide 8