
自秋标转材 Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 10 Alkynes Key Notes Electronic Structure;Acidity;Terminalalkynes By Junru Wang Email:wangjr07@163.com
By Junru Wang Email: wangjr07@163.com Chapter 10 Alkynes Organic Chemistry, 6th Edition L. G. Wade, Jr. Key Notes Electronic Structure; Acidity; Terminal alkynes
自秋不转大对 CONTENTS ■Properties Preparation ■Reactions of alkynes Electrophilic additions to alkynes Reduction Oxidation of alkynes OSpecial reactions of terminal alkynes
CONTENTS ◼Properties ◼Preparation ◼Reactions of alkynes ⚫Electrophilic additions to alkynes ⚫Reduction & Oxidation of alkynes ⚫Special reactions of terminal alkynes

Sec 1 Introduction Properties Alkynes contain a triple bond. General formula is C,H2m2. Two elements of unsaturation for each triple bond. Some reactions are like alkenes:addition and oxidation. Some reactions are specific to alkynes
Sec 1 Introduction & Properties ◼Alkynes contain a triple bond. ◼General formula is CnH2n-2 . ◼Two elements of unsaturation for each triple bond. ◼Some reactions are like alkenes: addition and oxidation. ◼Some reactions are specific to alkynes

自秋东转试于 Physical Properties Nonpolar,insoluble in water. Soluble in most organic solvents. Boiling points similar to alkane of same size. Less dense than water. Up to 4 carbons,gas at room temperature
Physical Properties ◼Nonpolar, insoluble in water. ◼Soluble in most organic solvents. ◼Boiling points similar to alkane of same size. ◼Less dense than water. ◼Up to 4 carbons, gas at room temperature
自秋特大材 Acetylene Acetylene is used in welding torches. In pure oxygen,temperature of flame reaches 2800C. It would violently decompose to its elements,but the cylinder on the torch contains crushed firebricki耐火砖粉末wet with acetone to moderate it
Acetylene ◼Acetylene is used in welding torches. ◼In pure oxygen, temperature of flame reaches 2800C. ◼It would violently decompose to its elements, but the cylinder on the torch contains crushed firebrick耐火砖粉末wet with acetone to moderate it
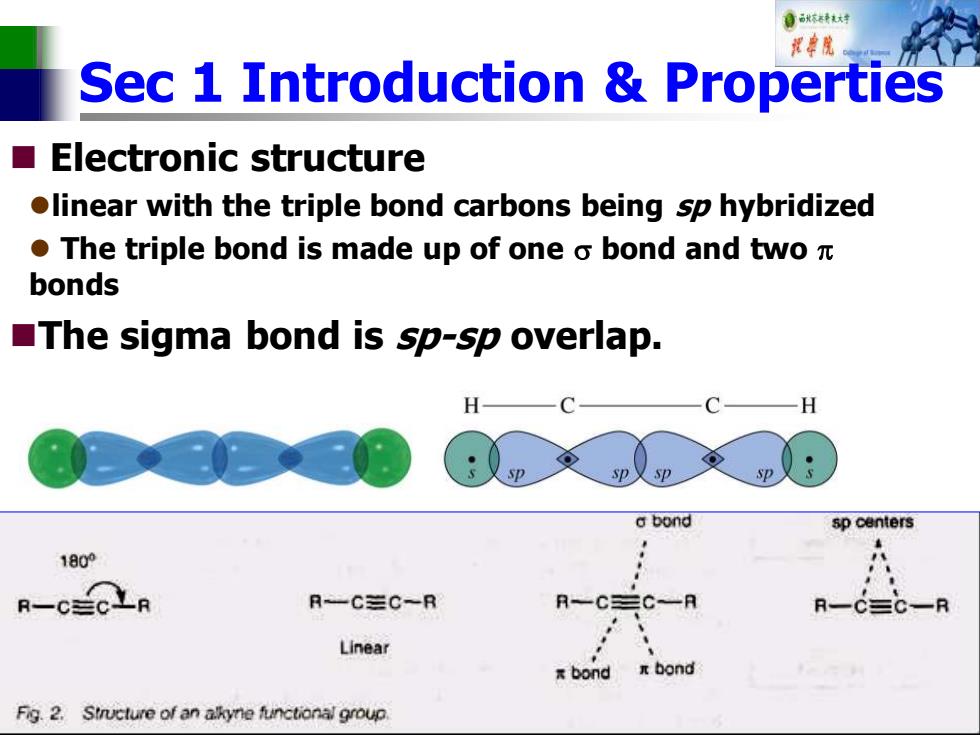
自秋转达对 视中院 Sec 1 Introduction Properties Electronic structure olinear with the triple bond carbons being sp hybridized ●The triple bond is made up of one o bond and two元 bonds The sigma bond is sp-sp overlap. H a bond sp centers 1800 R-CECLR R-C三C~R R-CEC-R Linear xbond Fig.2.Structure of an akyne functional group
Sec 1 Introduction & Properties ◼ Electronic structure ⚫linear with the triple bond carbons being sp hybridized ⚫ The triple bond is made up of one bond and two bonds ◼The sigma bond is sp-sp overlap
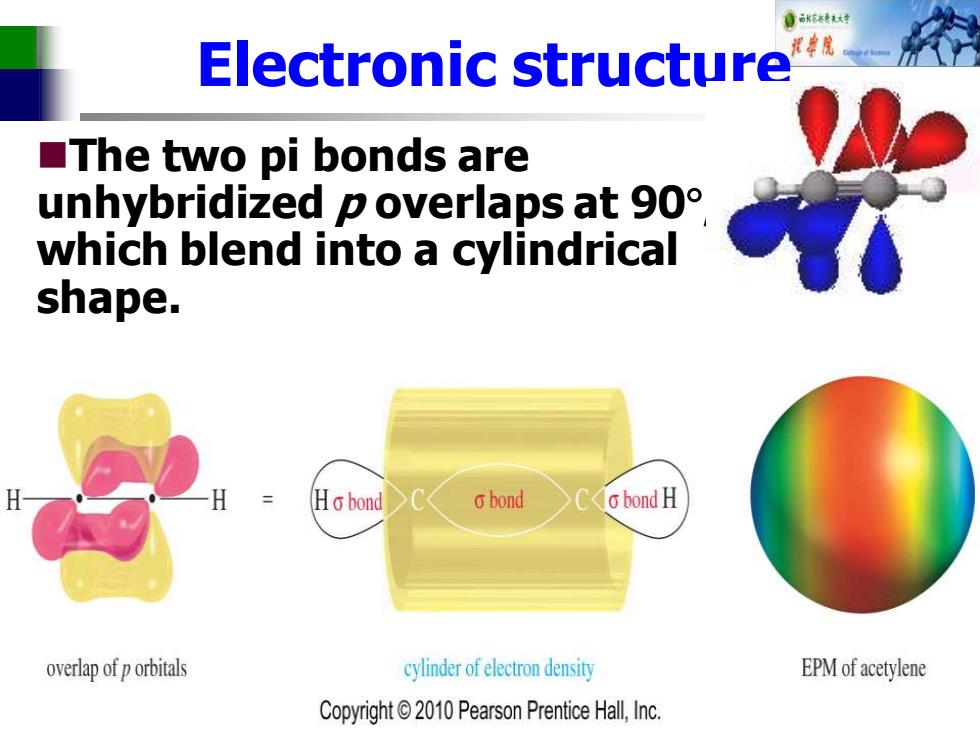
自标特花对 Electronic structure ■The two pi bonds are unhybridized p overlaps at 90 which blend into a cylindrical shape. Ho bond 6bond o bond H overlap of p orbitals cylinder of electron density EPM of acetylene Copyright2010 Pearson Prentice Hall,Inc
Electronic structure ◼The two pi bonds are unhybridized p overlaps at 90 , which blend into a cylindrical shape

自秋不转大对 Bond Lengths More s character,so shorter length. Three bonding overlaps,so shorter. 1.54A 1.33 1.20A H H H H一CC一H H H H H 1.09A 1.08A 1.06A ethane ethene ethyne Bond angle is180°,so linear geometry
Bond Lengths ◼More s character, so shorter length. ◼Three bonding overlaps, so shorter. Bond angle is 180, so linear geometry
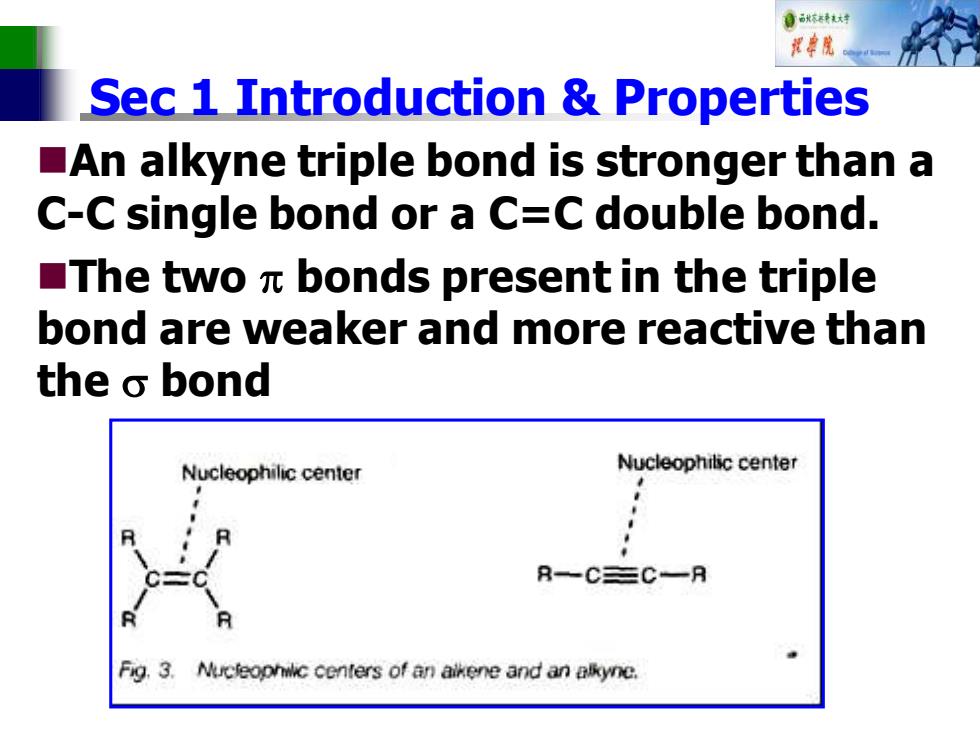
自秋特大中 花率院 Sec 1 Introduction Properties An alkyne triple bond is stronger than a C-C single bond or a C=C double bond. ■The twoπbonds present in the triple bond are weaker and more reactive than the o bond Nucleophilic center Nucleophilic center R一C三C一月 Fig.3. Nucteophilic centers of an aikene and an alkyne
Sec 1 Introduction & Properties ◼An alkyne triple bond is stronger than a C-C single bond or a C=C double bond. ◼The two bonds present in the triple bond are weaker and more reactive than the bond
自东精对 Acidity of Alkynes Terminal alkynes,R-C=C-H,are more acidic than other hydrocarbons. ■Acetylene-→acetylide by NH2,but not by OH-or RO-. More s character,so pair of electrons in anion is held more closely to the nucleus. Less charge separation,so more stable
Acidity of Alkynes ◼Terminal alkynes, R-CC-H, are more acidic than other hydrocarbons. ◼Acetylene → acetylide by NH2 - , but not by OH- or RO- . ◼More s character, so pair of electrons in anion is held more closely to the nucleus. Less charge separation, so more stable