
Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 23 Condensation and Alpha Substitution of Carbonyl Compounds
Chapter 23 Condensation and Alpha Substitution of Carbonyl Compounds Organic Chemistry, 6th Edition L. G. Wade, Jr
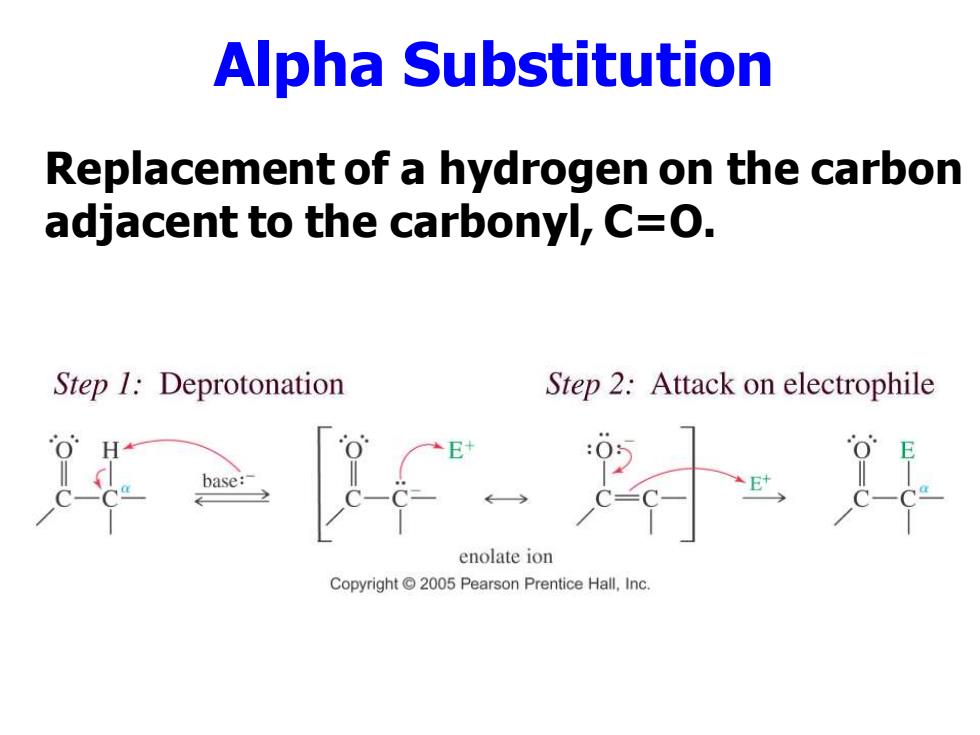
Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,C=O. Step 1:Deprotonation Step 2:Attack on electrophile base: enolate ion Copyright 2005 Pearson Prentice Hall,Inc
Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl, C=O
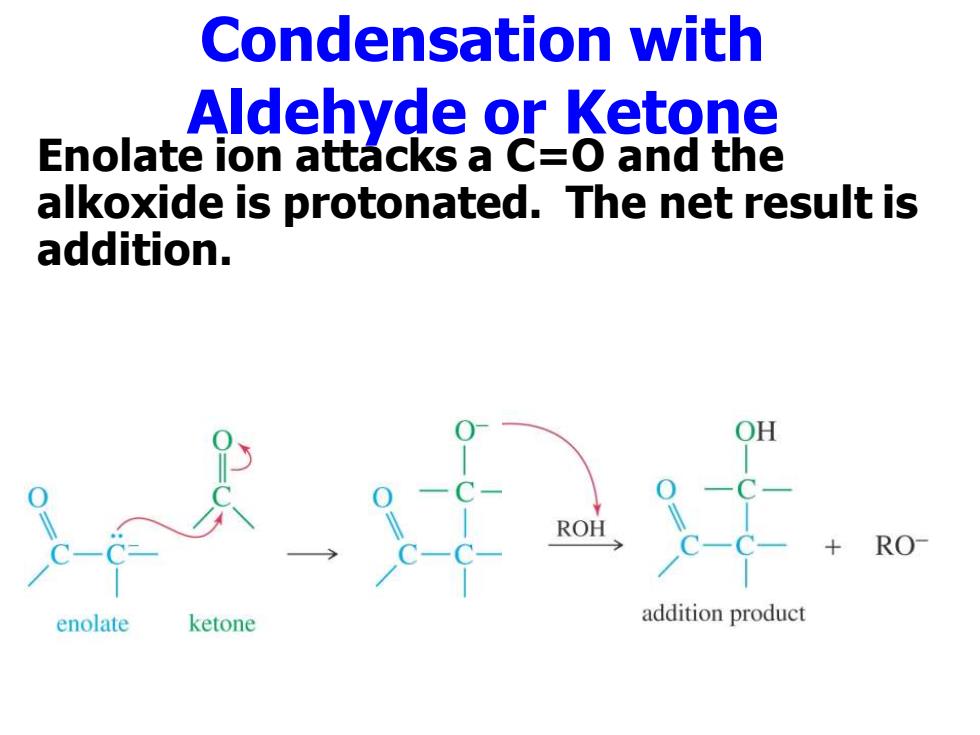
Condensation with Aldehyde or Ketone Enolate ion attacks a C=O and the alkoxide is protonated.The net result is addition. RO enolate ketone addition product
Condensation with Aldehyde or Ketone Enolate ion attacks a C=O and the alkoxide is protonated. The net result is addition
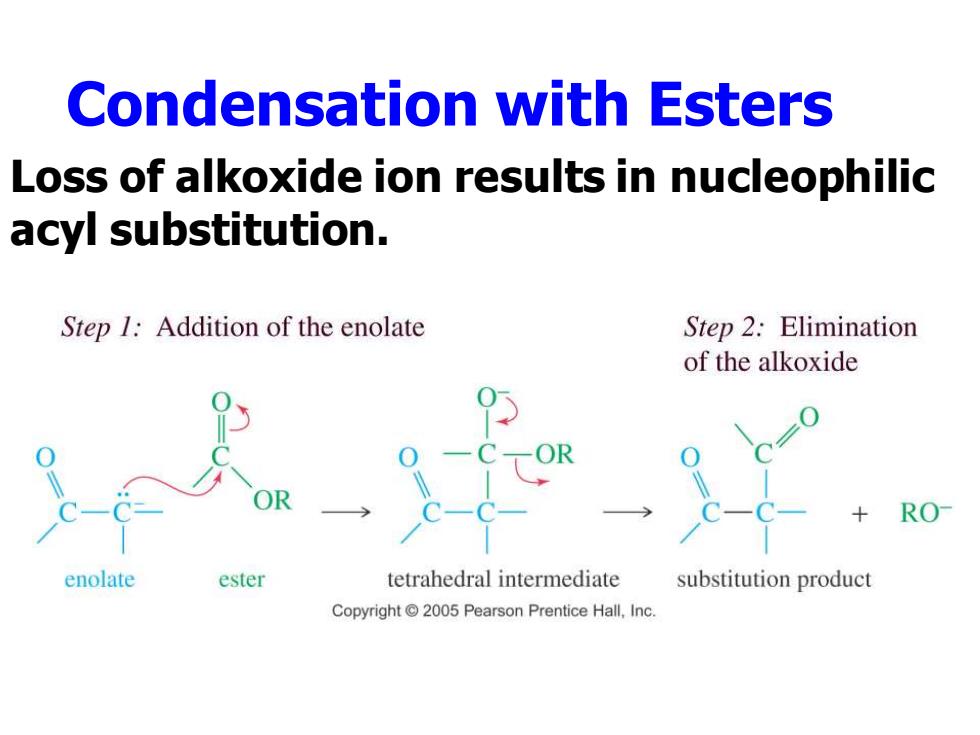
Condensation with Esters Loss of alkoxide ion results in nucleophilic acyl substitution. Step 1:Addition of the enolate Step 2:Elimination of the alkoxide RO enolate ester tetrahedral intermediate substitution product Copyright 2005 Pearson Prentice Hall,Inc
Condensation with Esters Loss of alkoxide ion results in nucleophilic acyl substitution
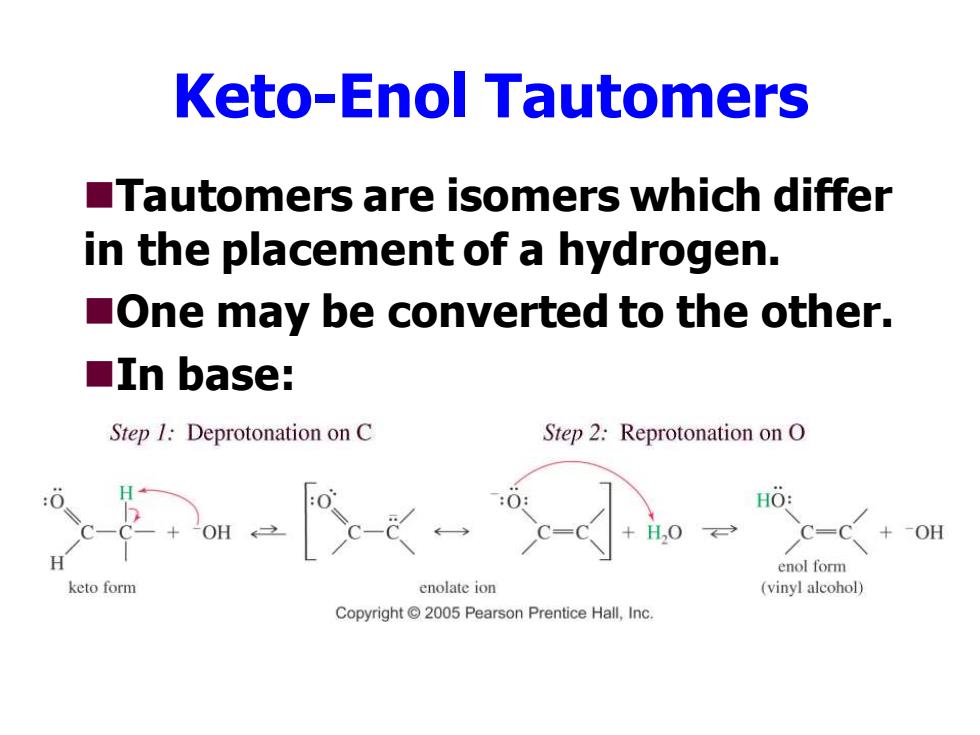
Keto-Enol Tautomers Tautomers are isomers which differ in the placement of a hydrogen. One may be converted to the other. ■In base: Step 1:Deprotonation on C Step 2:Reprotonation on O HO: DH +-OH enol form keto form enolate ion (vinyl alcohol) Copyright 2005 Pearson Prentice Hall,Inc
Keto-Enol Tautomers ◼Tautomers are isomers which differ in the placement of a hydrogen. ◼One may be converted to the other. ◼In base:
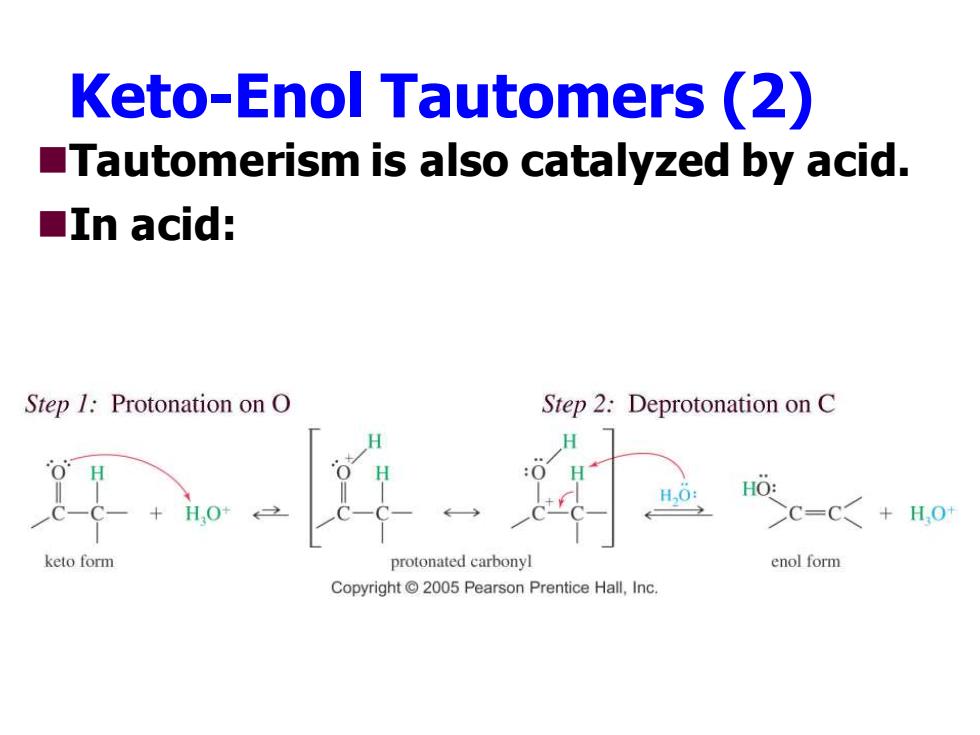
Keto-Enol Tautomers(2) Tautomerism is also catalyzed by acid. ■In acid: Step 1:Protonation on O Step 2:Deprotonation on C H H,0: HO: H,O+ C=C+H,0 keto form protonated carbonyl enol form Copyright 2005 Pearson Prentice Hall,Inc
Keto-Enol Tautomers (2) ◼Tautomerism is also catalyzed by acid. ◼In acid:
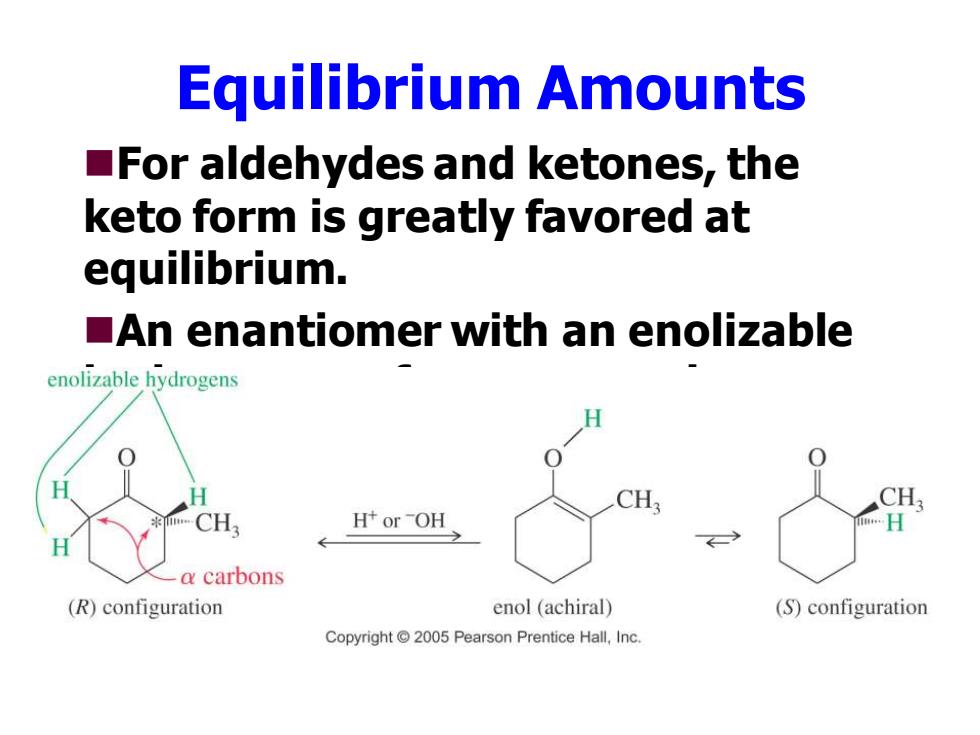
Equilibrium Amounts For aldehydes and ketones,the keto form is greatly favored at equilibrium. An enantiomer with an enolizable enolizable hydrogens CH: CH CH, H+orOH -a carbons (R)configuration enol (achiral) (S)configuration Copyright 2005 Pearson Prentice Hall,Inc
Equilibrium Amounts ◼For aldehydes and ketones, the keto form is greatly favored at equilibrium. ◼An enantiomer with an enolizable hydrogen can form a racemic mixture

Acidity of a-Hydrogens p for a-H of aldehyde or ketone ~20. Much more acidic than alkane or alkene(pK>40)or alkyne (pK =25). Less acidic than water (p=15.7)or alcohol (p 16-19). In the presence of hydroxide or alkoxide ions,only a small amount of enolate ion is present at equilibrium
Acidity of -Hydrogens ◼pKa for -H of aldehyde or ketone ~20. ◼Much more acidic than alkane or alkene (pKa > 40) or alkyne (pKa = 25). ◼Less acidic than water (pKa = 15.7) or alcohol (pKa = 16-19). ◼In the presence of hydroxide or alkoxide ions, only a small amount of enolate ion is present at equilibrium
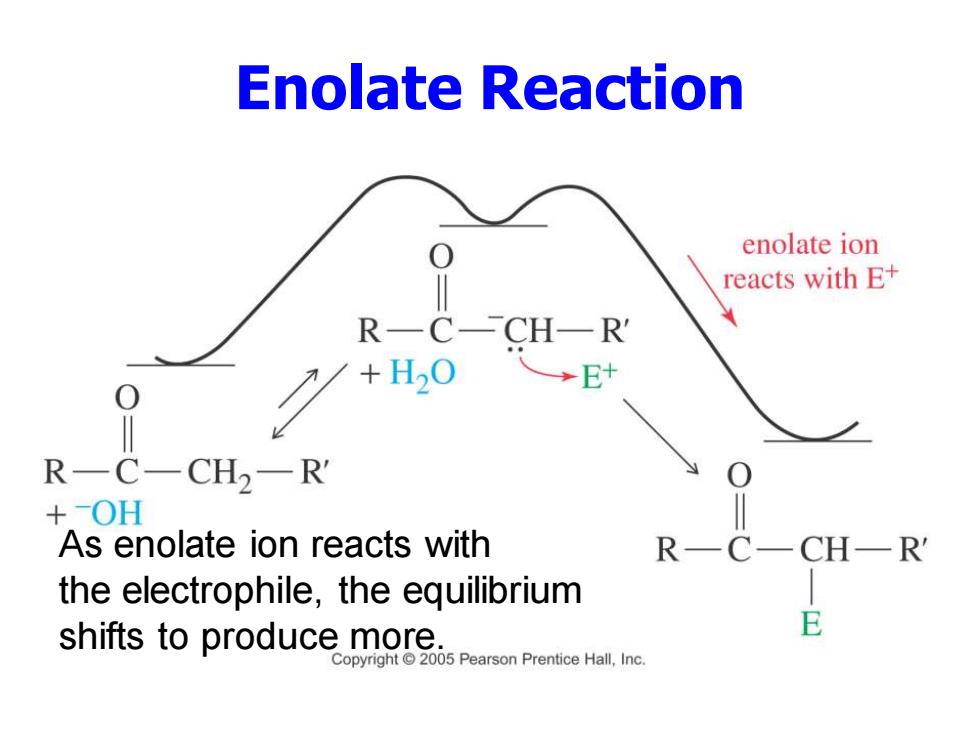
Enolate Reaction enolate ion reacts with E+ R—C-CH—R →E R一C一CH2一R +OH As enolate ion reacts with R一 the electrophile,the equilibrium shifts to produce more. Copyright 2005 Pearson Prentice Hall,Inc
Enolate Reaction As enolate ion reacts with the electrophile, the equilibrium shifts to produce more
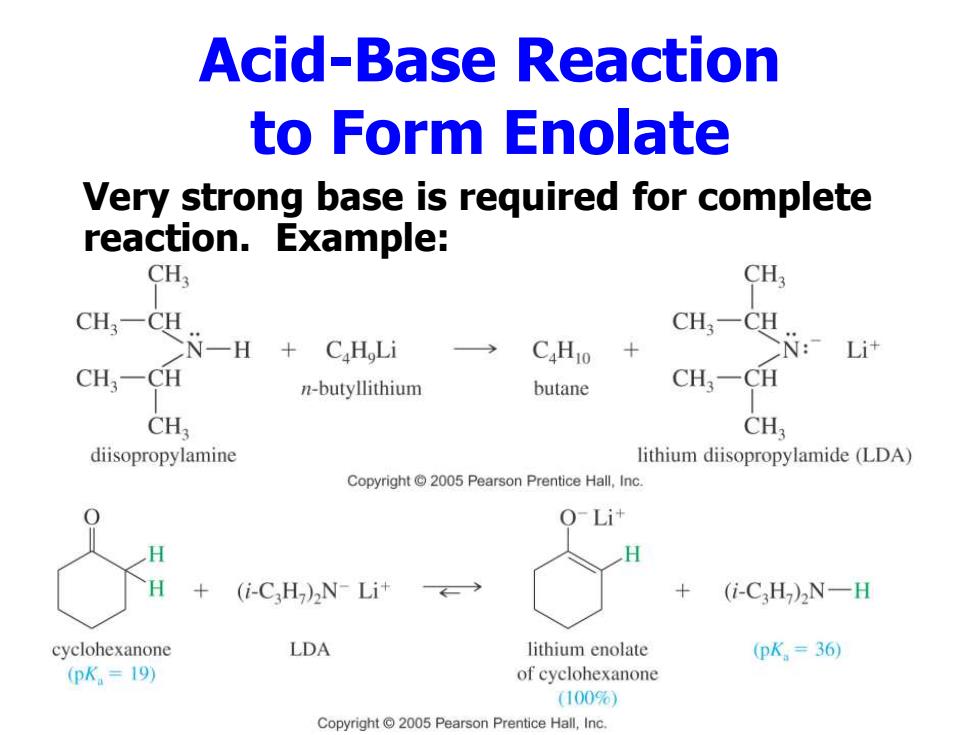
Acid-Base Reaction to Form Enolate Very strong base is required for complete reaction.Example: CH3 CH3 CH3-CH CH3-CH N一H CaH Li C.Hio N: Li计 CH-CH n-butyllithium butane CH3一CH CH, CH; diisopropylamine lithium diisopropylamide (LDA) Copyright 2005 Pearson Prentice Hall,Inc. (i-CH)2N-Li+ (i-C2H)2N-H cyclohexanone LDA lithium enolate (pK,=36 (pK.=19) of cyclohexanone (100%) Copyright 2005 Pearson Prentice Hall,Inc
Acid-Base Reaction to Form Enolate Very strong base is required for complete reaction. Example: