
Organic Chemistry with BiologicalApplications,3rEdition John McMurry Chapter 1 Structure and Bonding To review some ideas about atoms,bonds, and molecular geometry Junru Wang College of Chemistry and Pharmacy,Room C206,Science Building Tel:87092829(O):15109273921;Email:wangjunru@nwafu.edu.cn
Chapter 1 Structure and Bonding Organic Chemistry with Biological Applications, 3 rd Edition John McMurry To review some ideas about atoms, bonds, and molecular geometry Junru Wang College of Chemistry and Pharmacy,Room C206, Science Building Tel: 87092829(O);15109273921; Email: wangjunru@nwafu.edu.cn

Organic Chemistry with BiologicalApplications,3Edition 化学 John McMurry Key Notes Electronic Configurations,Covalent Bonds, Chemical Bonding theory,Structure Drawing Homework:p161-10,1-11;P27c1-36,1-42 By Junru Wang College of Chemistry and Pharmacy Room C206,Science Building Tel:87092829(O):Email:wangjr07@163.com
By Junru Wang College of Chemistry and Pharmacy Room C206, Science Building Tel: 87092829(O);Email: wangjr07@163.com Key Notes Electronic Configurations, Covalent Bonds, Chemical Bonding theory, Structure Drawing Organic Chemistry with Biological Applications, 3 rd Edition John McMurry Homework: p16 1-10,1-11;P27c 1-36,1-42
Main Contents -Atomic structure -Development of chemical bonding theory -Hybridization theory -Drawing chemical structures
Main Contents ▪Atomic structure ▪Development of chemical bonding theory ▪Hybridization theory ▪Drawing chemical structures
SEC 1 Definitions Organic Chemistry What:The study of carbon-containing compounds Why:Pervasive in nature 普逼深入的 Chemical foundation of biology Improve standard of living(medicines,plastics,pesticides..) How:Examine structure and analyze how it governs reactivity
普遍深入的 SEC 1 Definitions

Almost everything you see in this picture is made of organic chemicals
Almost everything you see in this picture is made of organic chemicals
History...Key time -Organic Chemistry is... Old:“derived from living organisms” ·New:“chemistry of carbon compoundsi” From inorganic to organic,Wohler,1828 In 1770,Swedish chemist Torberm Bergman was the first to express the difference between "organic"and “inorganic'”substances Organic---derived from living organism Originally,Study of compounds extracted from living organisms and their natural products INORGANIC CHEMICALS were found primarily in the earth as mineral deposits,but could also be prepared by man
History… Key time ▪Organic Chemistry is… ▪ Old: “derived from living organisms” ▪ New: “chemistry of carbon compounds” ▪ From inorganic to organic, Wöhler, 1828 ▪ In 1770, Swedish chemist Torberm Bergman was the first to express the difference between “organic” and “inorganic” substances Organic --- derived from living organism ▪ Originally, Study of compounds extracted from living organisms and their natural products INORGANIC CHEMICALS were found primarily in the earth as mineral deposits, but could also be prepared by man

History...VITALISM 神秘莫测的力 Vital force theory:生命力学说 Compounds such as sugar,urea,starch,waxes, and plant oils were considered“organic” -People believed that...... Organic compounds needs a "vital force"to create them Vitalism was the belief that certain chemicals, ORGANIC CHEMICALS,could only be made by living organisms
History… VITALISM 神秘莫测的力 Vital force theory:生命力学说 ▪ Compounds such as sugar, urea, starch, waxes, and plant oils were considered “organic” ▪ People believed that…… ▪ Organic compounds needs a “vital force” to create them ▪ Vitalism was the belief that certain chemicals, ORGANIC CHEMICALS, could only be made by living organisms
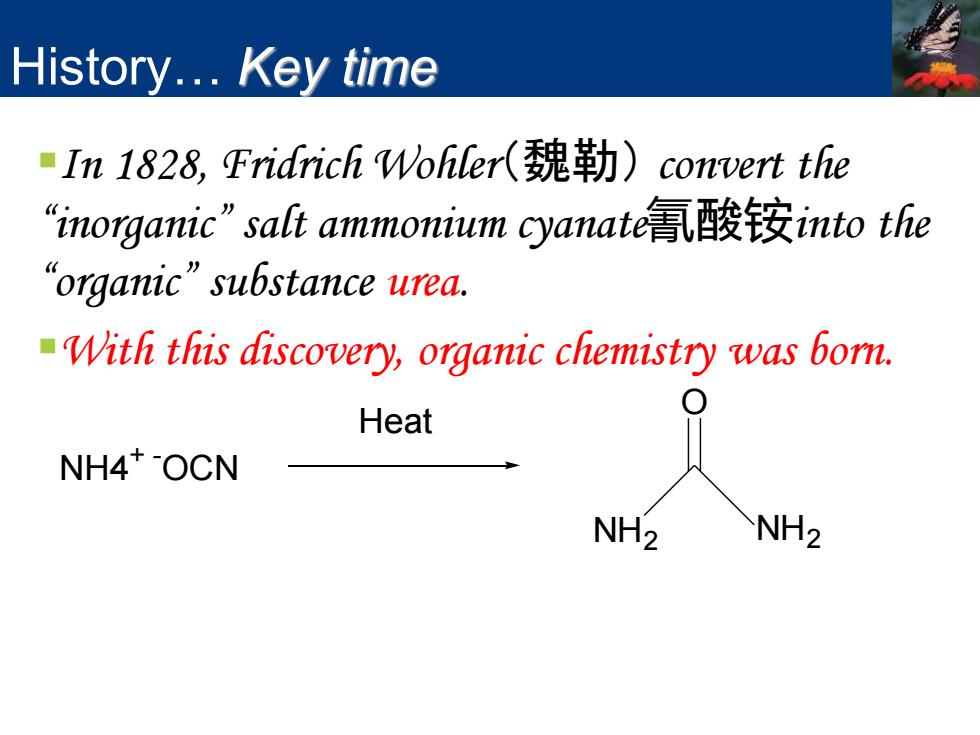
History...Key time In1828,Fridrich Wohler(魏勒) convert the “inorganic”salt ammonium cyanate氰酸铵into the “organic”su6 stance urea.. With this discovery,organic chemistry was bom. Heat NH4*OCN NH2 NH2
History… Key time ▪In 1828, Fridrich Wohler(魏勒) convert the “inorganic” salt ammonium cyanate氰酸铵into the “organic” substance urea. ▪With this discovery, organic chemistry was born. NH4 + - OCN NH2 O NH2 Heat

Unique property of carbon --Bond to one another in a virtually limitless number of arrangements Carbon Atomic Weight 12.011 Melting Point 3823.2K Boiling Point 1st lonization Energy 1086.4 kJ/mol Density 2.266gcm3 Electron Configuration [He]2s22p2 Atomic Radius 77.2
Unique property of carbon ---Bond to one another in a virtually limitless number of arrangements
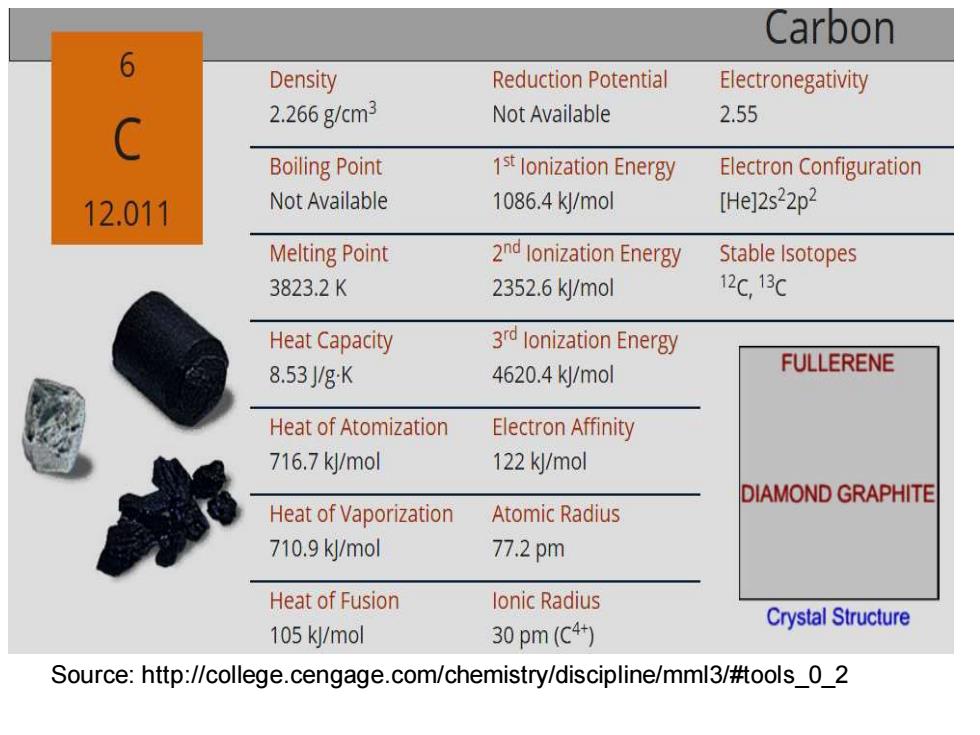
Carbon 6 Density Reduction Potential Electronegativity 2.266g/cm3 Not Available 2.55 Boiling Point 1st lonization Energy Electron Configuration 12.011 Not Available 1086.4k/mol [He]2s22p2 Melting Point 2nd lonization Energy Stable Isotopes 3823.2K 2352.6k/mol 12C,13C Heat Capacity 3rd lonization Energy 8.53/gK 4620.4k/mol FULLERENE Heat of Atomization Electron Affinity 716.7k/mol 122 kl/mol DIAMOND GRAPHITE Heat of Vaporization Atomic Radius 710.9k/mol 77.2pm Heat of Fusion lonic Radius Crystal Structure 105 kJ/mol 30pm(C4) Source:http://college.cengage.com/chemistry/discipline/mml3/#tools 0 2
Source: http://college.cengage.com/chemistry/discipline/mml3/#tools_0_2