
Organic Chemistry with BiologicalApplications,3Edition John McMurry Chapter 03 Organic compounds: alkanes and their stereochemistry Alkanes are relatively unreactive with onty a minor role in a few biological processes. This chap will provide some of 3D aspects of molecules,with a topic of particular importance in understanding biological organic chemistry
Chapter 03 Organic compounds : alkanes and their stereochemistry Organic Chemistry with Biological Applications, 3 rd Edition John McMurry Alkanes are relatively unreactive with only a minor role in a few biological processes. This chap will provide some of 3D aspects of molecules, with a topic of particular importance in understanding biological organic chemistry

Organic Chemistry with BiologicalApplications,3rEdition John McMurry Chapter 03 Organic compounds: alkanes and their stereochemistry Key Notes Conformation;Conformers;Isomer Radical RXN
Chapter 03 Organic compounds : alkanes and their stereochemistry Key Notes Conformation; Conformers; Isomer; Radical RXN Organic Chemistry with Biological Applications, 3 rd Edition John McMurry
Main Contents Alkanes and Alkane Isomers ■Alkyl Groups Properties of Alkanes ■Conformations √Ethane √Other alkanes A membrane channel protein that conducts K+ions across cell membranes
Main Contents ◼Alkanes and Alkane Isomers ◼Alkyl Groups ◼Properties of Alkanes ◼Conformations ✓Ethane ✓Other alkanes A membrane channel protein that conducts K+ ions across cell membranes

Sec 1 Alkanes and Alkane Isomers Alkanes often described as saturated hydrocarbons Hydrocarbons Contain only carbon and hydrogen Saturated Contain maximum possible number of hydrogens per carbon and have only C-C and C-H single bonds Alkanes occasionally referred to as aliphatic compounds,a name derived from the Greek word aleiphas,meaning "fat" Fatty acyl Glycerol Stearoyl (stearic acid) CH2OCCH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH3 Oleoyl (oleic acid) CHOCCH2CH2CH2CH2CH2CH2CH2CH-CHCH2CH2CH2CH2CH2CH2CH2CH3 Linoleoyl (linoleic acid) CH2OCCH2CH2CH2CH2CH2CH2CH2CH=CHCH2CH-CHCH2CH2CH2CH2CH3 A triacylglycerol Cengnge Leaming All Righes Reporved
Alkanes often described as saturated hydrocarbons ▪ Hydrocarbons Contain only carbon and hydrogen ▪ Saturated ▪ Contain maximum possible number of hydrogens per carbon and have only C-C and C-H single bonds Alkanes occasionally referred to as aliphatic compounds, a name derived from the Greek word aleiphas, meaning “fat” Sec 1 Alkanes and Alkane Isomers
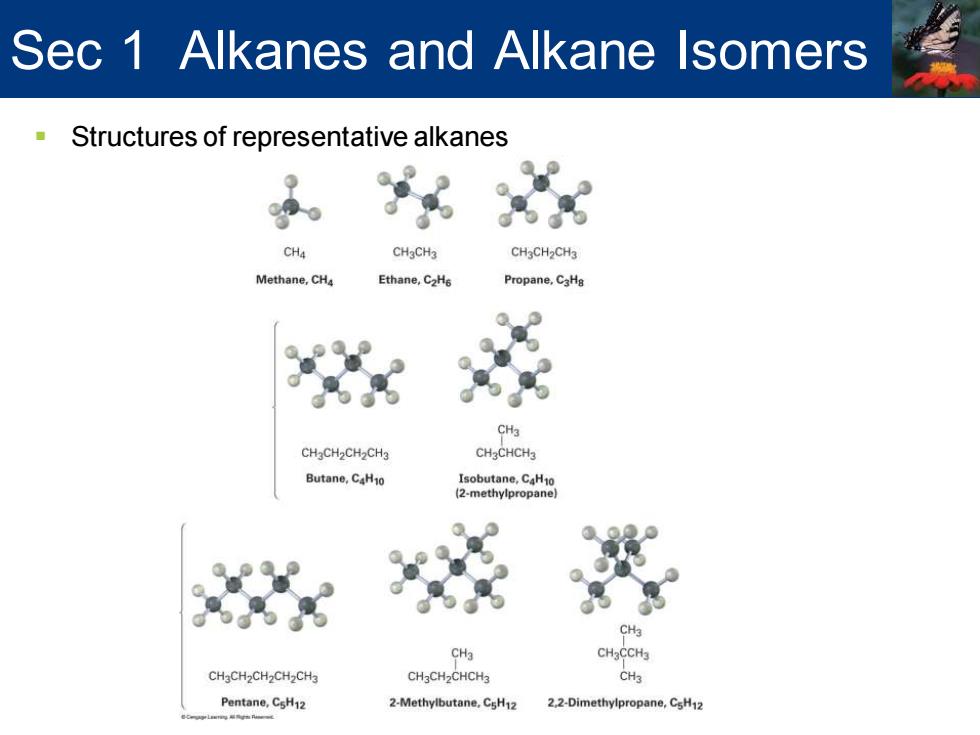
Sec 1 Alkanes and Alkane Isomers Structures of representative alkanes CH4 CHaCH3 CH3CH2CH3 Methane,CHa Ethane,C2H6 Propane,CaHg CH3 CHgCH2CH2CH3 CH2CHCH3 Butane,C4H10 Isobutane,CaH10 2-methylpropane】 C CH3 CHaCCHa CH3CH2CH2CH2CH3 CH3CH2CHCH3 CH3 Pentane,CsH12 2-Methylbutane.CsH12 2.2-Dimethylpropane,CsH12
▪ Structures of representative alkanes Sec 1 Alkanes and Alkane Isomers
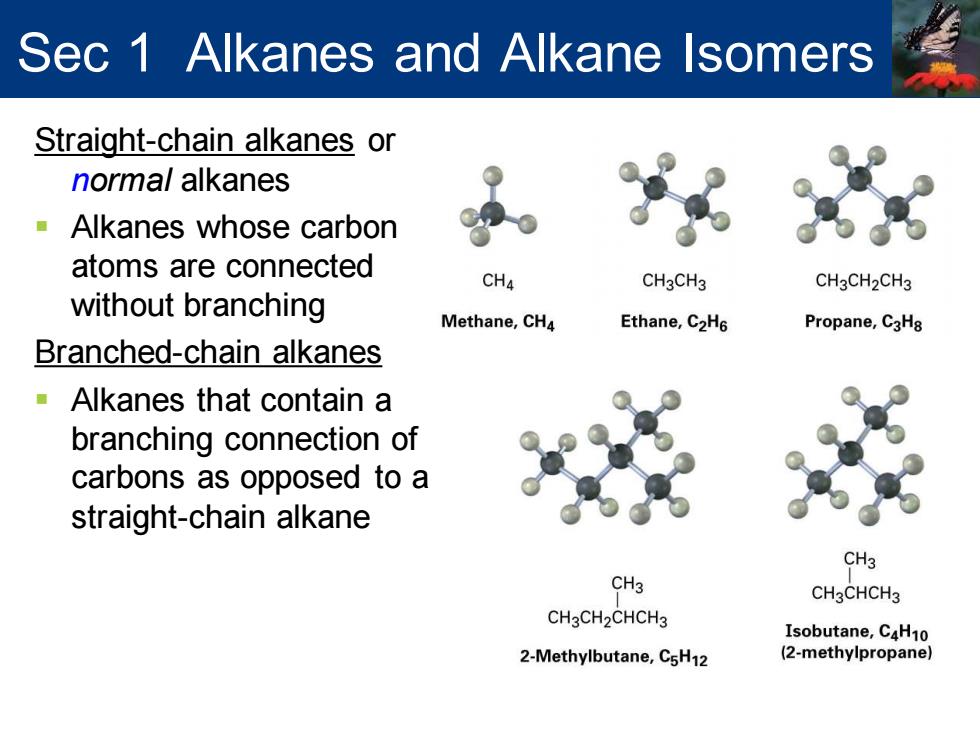
Sec 1 Alkanes and Alkane Isomers Straight-chain alkanes or normal alkanes -Alkanes whose carbon atoms are connected CH4 CH3CH3 CH3CH2CH3 without branching Methane,CH4 Ethane,C2H6 Propane,C3H8 Branched-chain alkanes Alkanes that contain a branching connection of carbons as opposed to a straight-chain alkane CH3 CH3 CH3CHCH3 CH3CH2CHCH3 Isobutane,C4H10 2-Methylbutane,C5H12 (2-methylpropane)
Straight-chain alkanes or normal alkanes ▪ Alkanes whose carbon atoms are connected without branching Branched-chain alkanes ▪ Alkanes that contain a branching connection of carbons as opposed to a straight-chain alkane Sec 1 Alkanes and Alkane Isomers
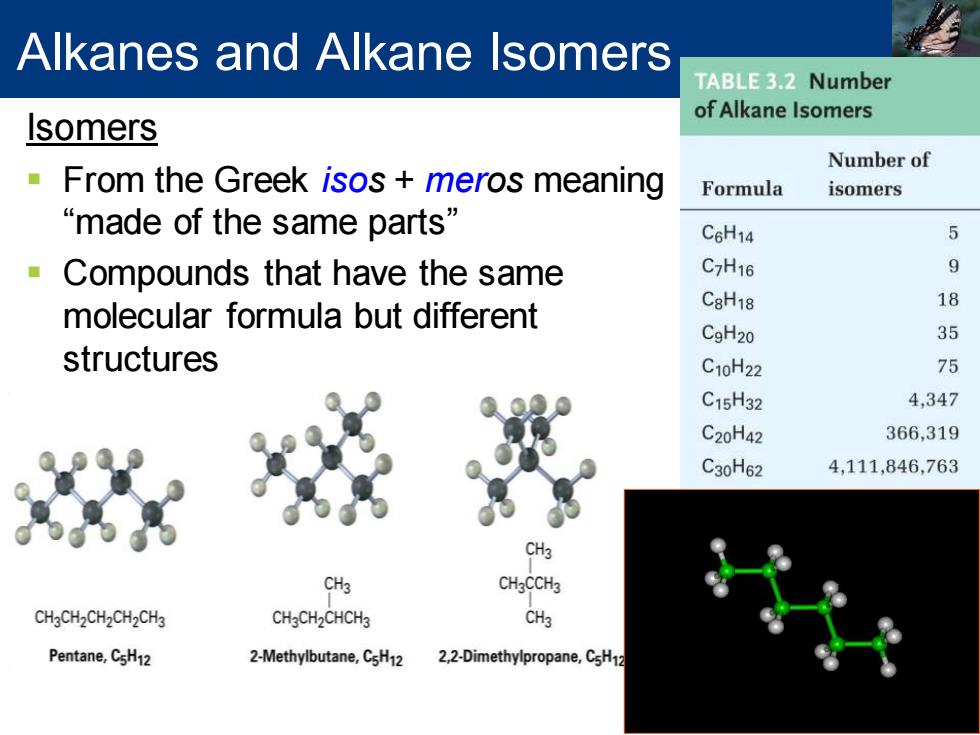
Alkanes and Alkane Isomers TABLE 3.2 Number of Alkane Isomers Isomers Number of From the Greek isos meros meaning Formula isomers “made of the same parts” C6H14 Compounds that have the same C7H16 molecular formula but different C8H18 18 CgH20 35 structures C10H22 75 C15H32 4,347 C20H42 366,319 C30H62 4,111,846,763 CH3 CH3 CH3CCH3 CH3CH2CH2CH2CH3 CH3CH2CHCH3 CH3 Pentane,CsH12 2-Methylbutane,CsH12 2,2-Dimethylpropane,CsH
Isomers ▪ From the Greek isos + meros meaning “made of the same parts” ▪ Compounds that have the same molecular formula but different structures Alkanes and Alkane Isomers
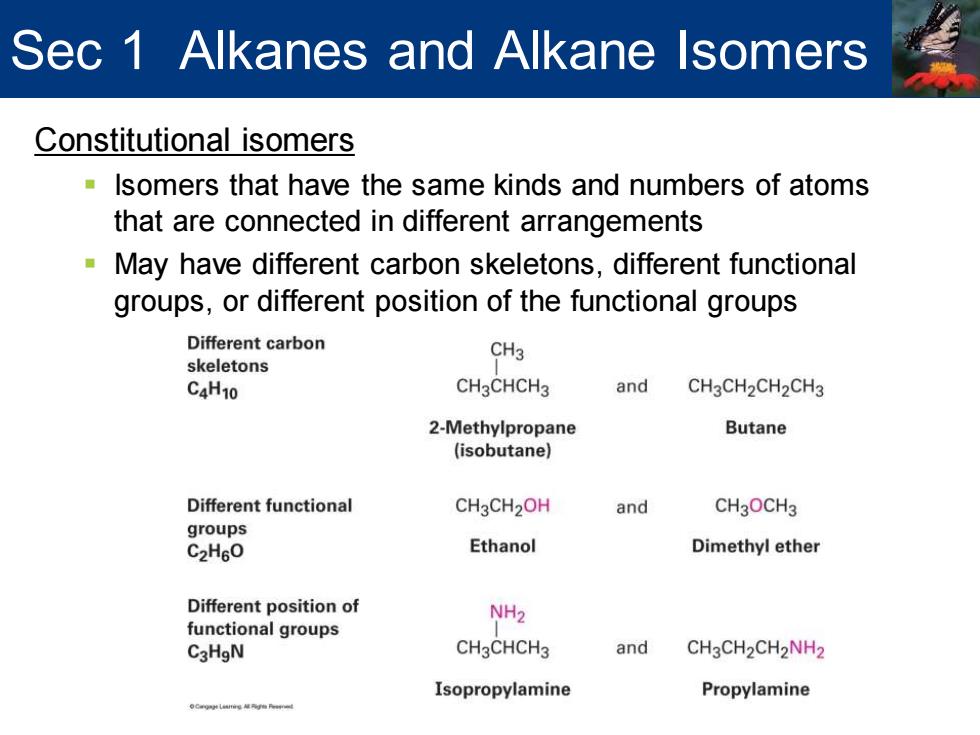
Sec 1 Alkanes and Alkane Isomers Constitutional isomers Isomers that have the same kinds and numbers of atoms that are connected in different arrangements May have different carbon skeletons,different functional groups,or different position of the functional groups Different carbon CH3 skeletons C4H10 CH3CHCH3 and CH3CH2CH2CH3 2-Methylpropane Butane (isobutane) Different functional CH3CH2OH and CH3OCH3 groups C2H60 Ethanol Dimethyl ether Different position of NH2 functional groups C3HgN CH3CHCH3 and CH3CH2CH2NH2 Isopropylamine Propylamine
Constitutional isomers ▪ Isomers that have the same kinds and numbers of atoms that are connected in different arrangements ▪ May have different carbon skeletons, different functional groups, or different position of the functional groups Sec 1 Alkanes and Alkane Isomers
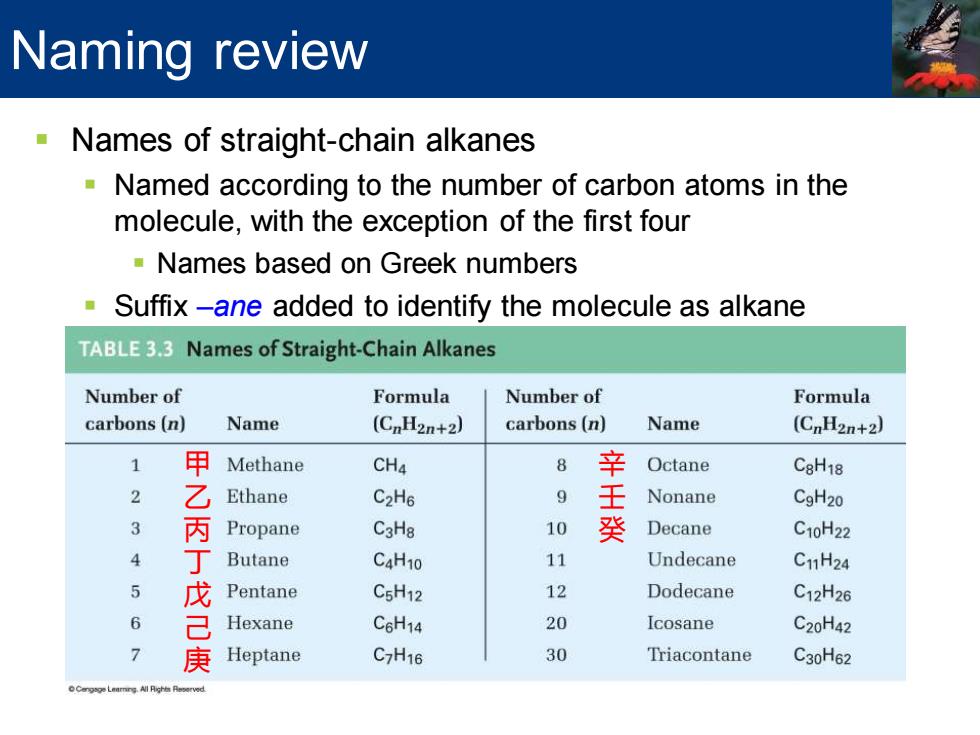
Naming review Names of straight-chain alkanes Named according to the number of carbon atoms in the molecule,with the exception of the first four -Names based on Greek numbers Suffix-ane added to identify the molecule as alkane TABLE3.3 Names of Straight-Chain Alkanes Number of Formula Number of Formula carbons(n) Name (CnH2n+2) carbons(n) Name (CnH2n+2) 1 甲 Methane CH4 8 辛 Octane C8H18 乙 Ethane C2H6 9 Nonane CgH20 3 Propane C3Ha 10 癸 Decane C10H22 ¥ 丁 Butane C4H10 11 Undecane C1H24 Pentane CsH12 12 Dodecane C12H26 6 己 Hexane C6H14 20 Icosane C20H42 7 庚 Heptane C7H16 30 Triacontane C30H62
▪ Names of straight-chain alkanes ▪ Named according to the number of carbon atoms in the molecule, with the exception of the first four ▪ Names based on Greek numbers ▪ Suffix –ane added to identify the molecule as alkane Naming review 甲 乙 丙 丁 戊 己 庚 辛 壬 癸

Major Uses of Alkanes -C1-C2:gases (natural gas) -C3-C4:liquified petroleum (LPG) -Cs-Ca:gasoline -C-C16:diesel,kerosene,jet fuel -C17-up:lubricating oils,heating oil -Origin:petroleum refining
Major Uses of Alkanes ▪C1 -C2 : gases (natural gas) ▪C3 -C4 : liquified petroleum (LPG) ▪C5 -C8 : gasoline ▪C9 -C16: diesel, kerosene, jet fuel ▪C17-up: lubricating oils, heating oil ▪Origin: petroleum refining