有机化学oRCAnI©cIem1STY 盖讲:王德儒87092829(0) 理学院寇化素理科楼2层06 Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter6 Stereochemistry
Chapter 6 Stereochemistry Organic Chemistry, 6th Edition L. G. Wade, Jr. 有机化学ORGANIC CHEMISTRY 主讲:王俊儒 87092829(O) 理学院应化系理科楼2层C206
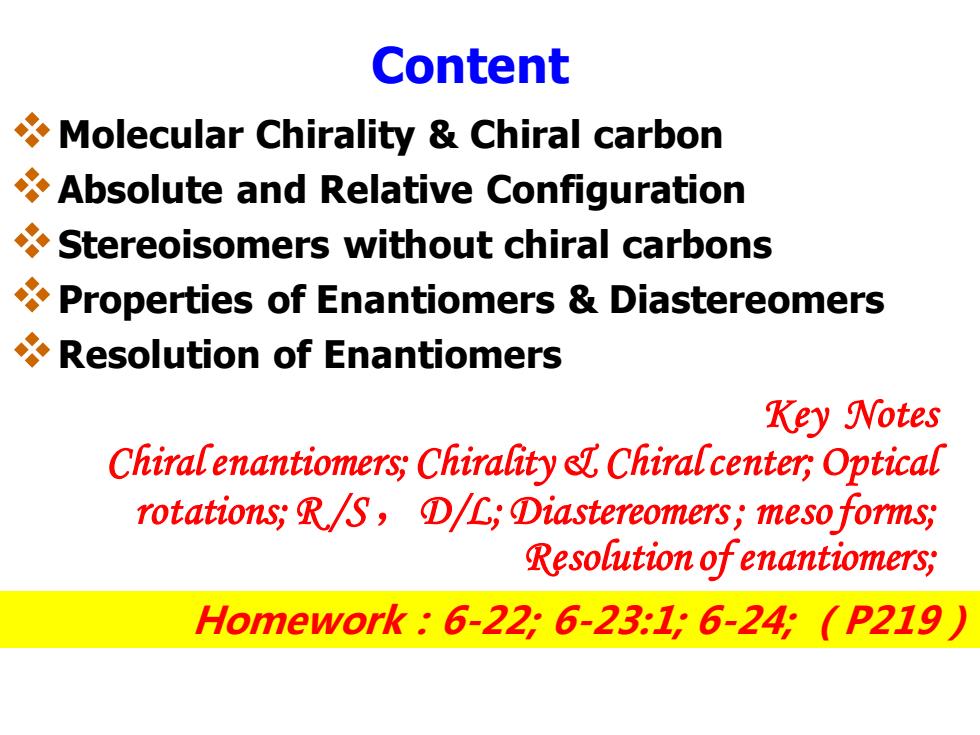
Content Molecular Chirality Chiral carbon Absolute and Relative Configuration Stereoisomers without chiral carbons Properties of Enantiomers Diastereomers Resolution of Enantiomers Key Notes Chiralenantiomers;Chirality e Chiralcenter;Optical rotations;R/S,①/L,①iastereomers;meso forms; Resolution of enantiomers; Homework:6-226-23:16-24(P219
Content ❖Molecular Chirality & Chiral carbon ❖Absolute and Relative Configuration ❖Stereoisomers without chiral carbons ❖Properties of Enantiomers & Diastereomers ❖Resolution of Enantiomers Key Notes Chiral enantiomers; Chirality & Chiral center; Optical rotations; R /S ,D/L; Diastereomers ; meso forms; Resolution of enantiomers; Homework:6-22; 6-23:1; 6-24; (P219)
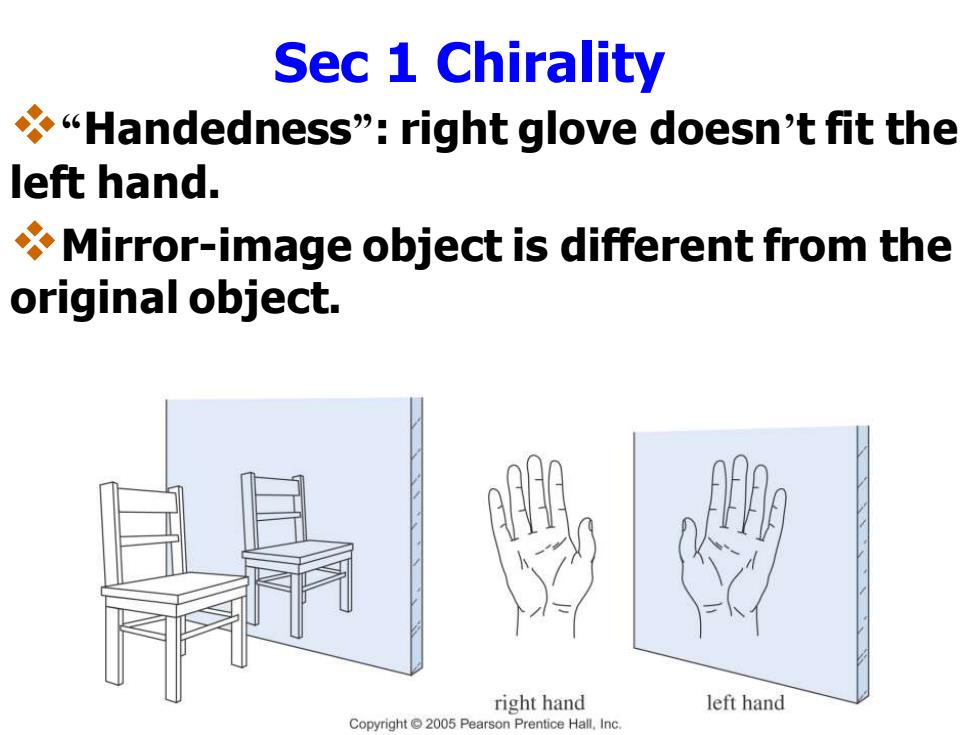
Sec 1 Chirality Handedness":right glove doesn't fit the left hand. Mirror-image object is different from the original object. right hand left hand Copyright2005 Pearson Prentice Hall.Inc
Sec 1 Chirality ❖“Handedness”: right glove doesn’t fit the left hand. ❖Mirror-image object is different from the original object
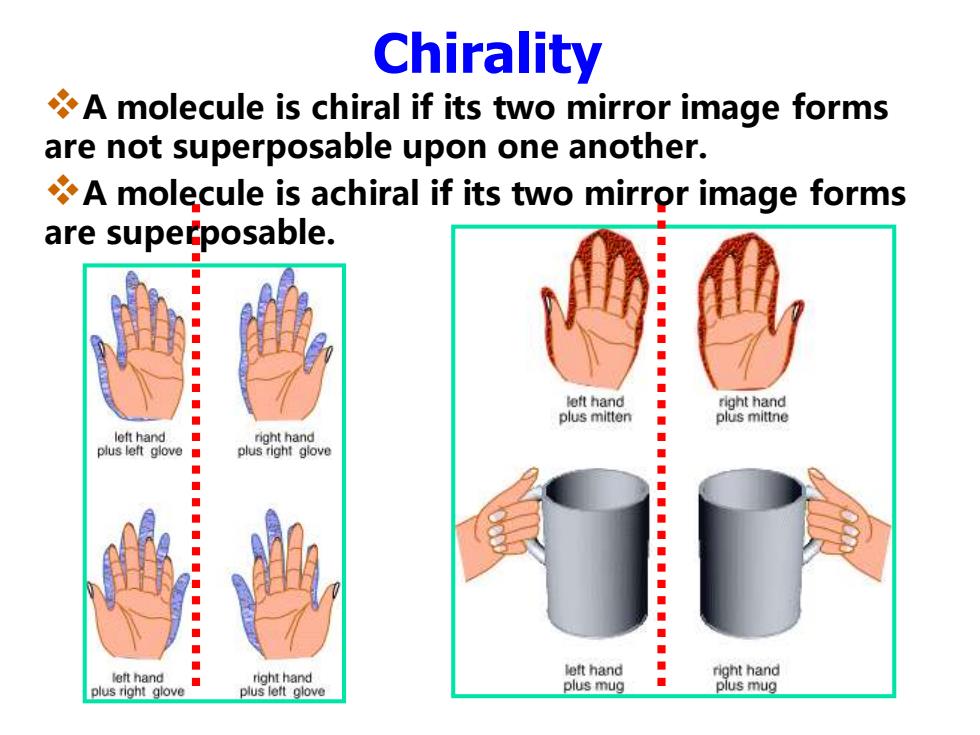
Chirality A molecule is chiral if its two mirror image forms are not superposable upon one another. A molecule is achiral if its two mirror image forms are superposable. ◆ left hand ◆ right hand plus mitten plus mittne left hand ◆ right hand ◆ plus left glove■ plus right glove ◆ ◆ ● ◆ ◆ ◆ ■ left hand right hand left hand right hand plus right glove ◆ plus left glove plus mug plus mug
Chirality ❖A molecule is chiral if its two mirror image forms are not superposable upon one another. ❖A molecule is achiral if its two mirror image forms are superposable
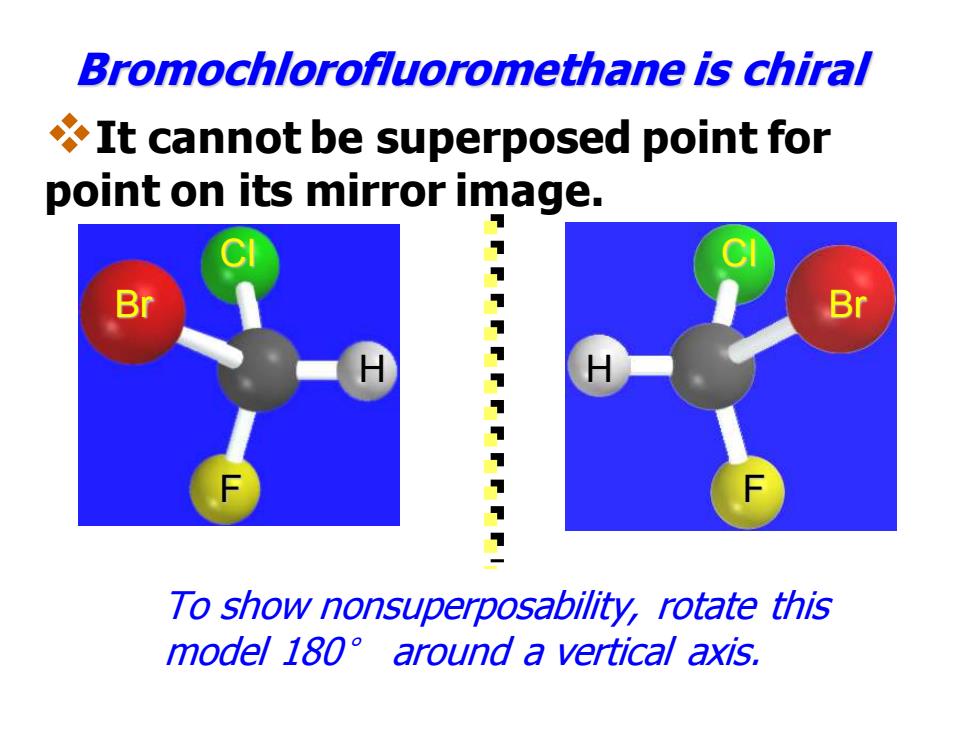
Bromochlorofluoromethane is chiral It cannot be superposed point for point on its mirror image. Br 7 To show nonsuperposability,rotate this model 180 around a vertical axis
Br Cl H F Bromochlorofluoromethane is chiral H Cl Br F To show nonsuperposability, rotate this model 180° around a vertical axis. ❖It cannot be superposed point for point on its mirror image
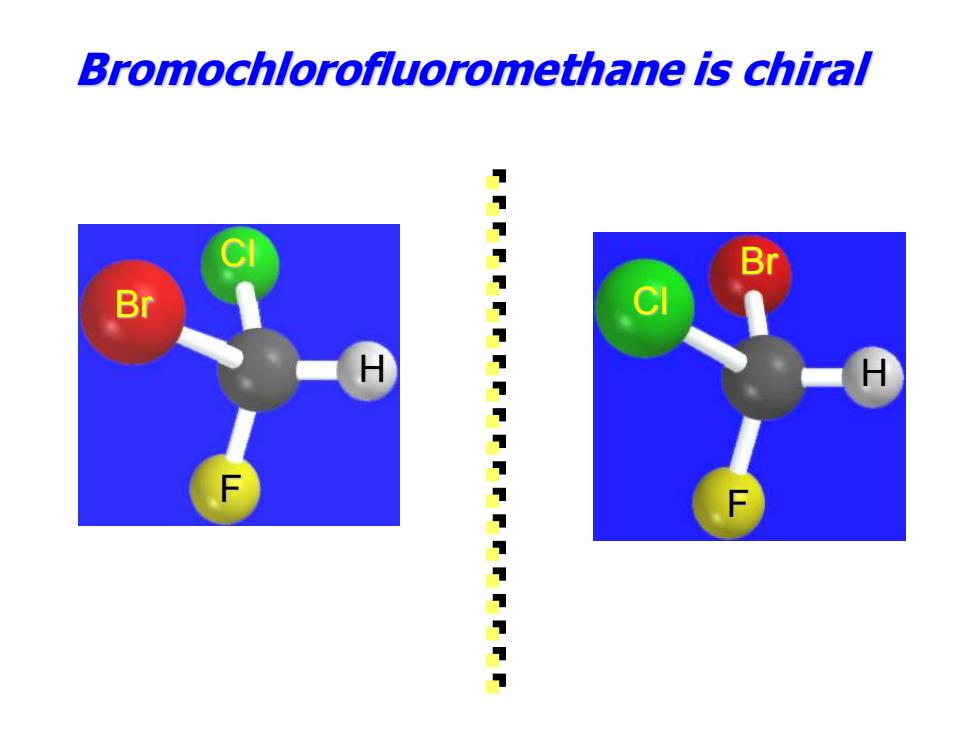
Bromochlorofluoromethane is chiral Br 11111111111111111111
Br Cl H F Bromochlorofluoromethane is chiral H Cl Br F
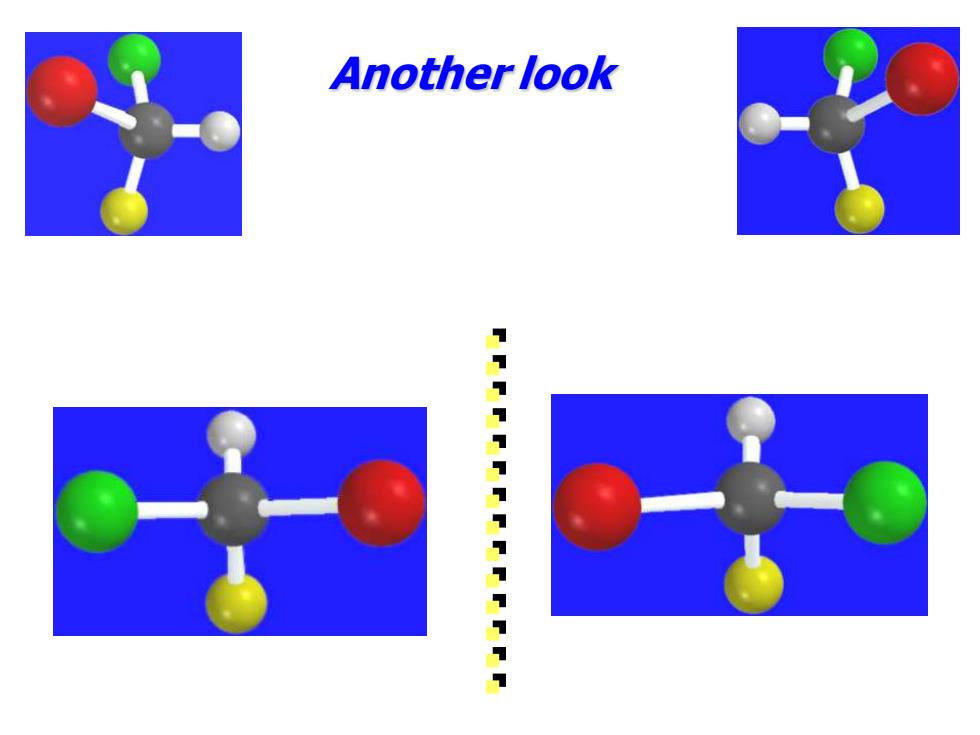
Another look 11111111111111
Another look
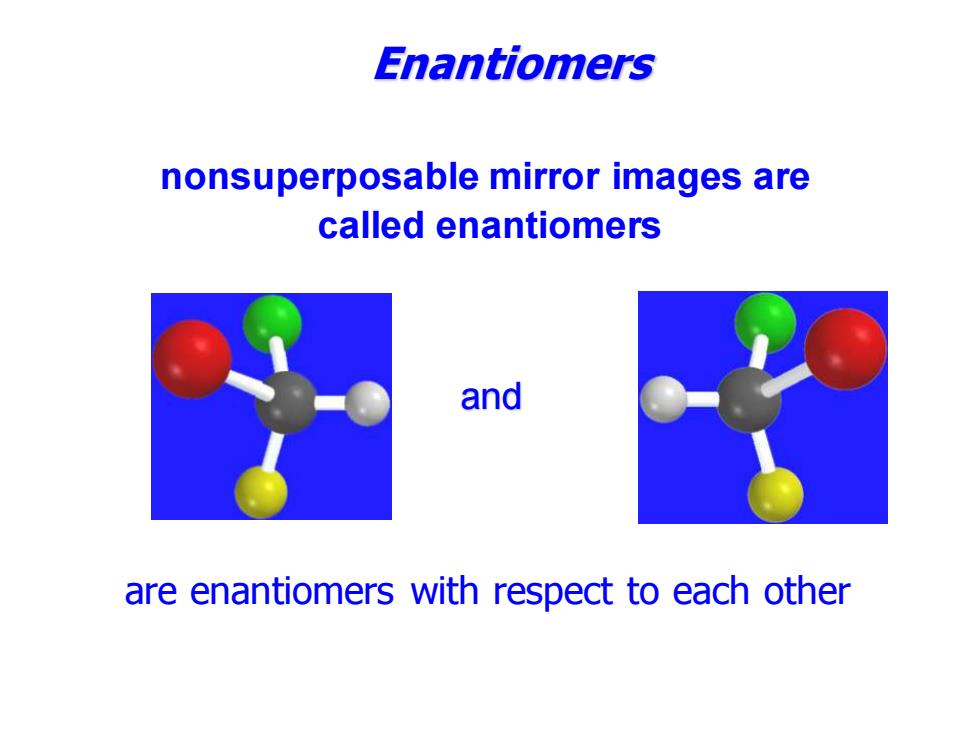
Enantiomers nonsuperposable mirror images are called enantiomers and are enantiomers with respect to each other
are enantiomers with respect to each other and nonsuperposable mirror images are called enantiomers Enantiomers
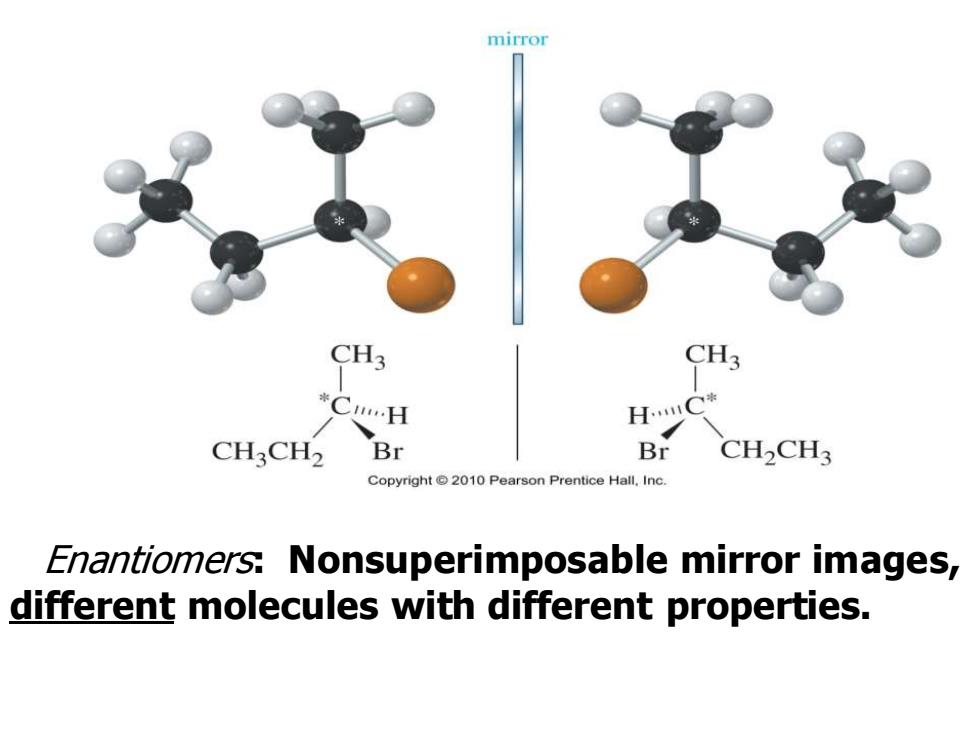
mirror CH3 CH3 *CH H.C* CH3CH2 Br Br CH2CH3 Copyright 2010 Pearson Prentice Hall.Inc. Enantiomers:Nonsuperimposable mirror images, different molecules with different properties
Enantiomers: Nonsuperimposable mirror images, different molecules with different properties
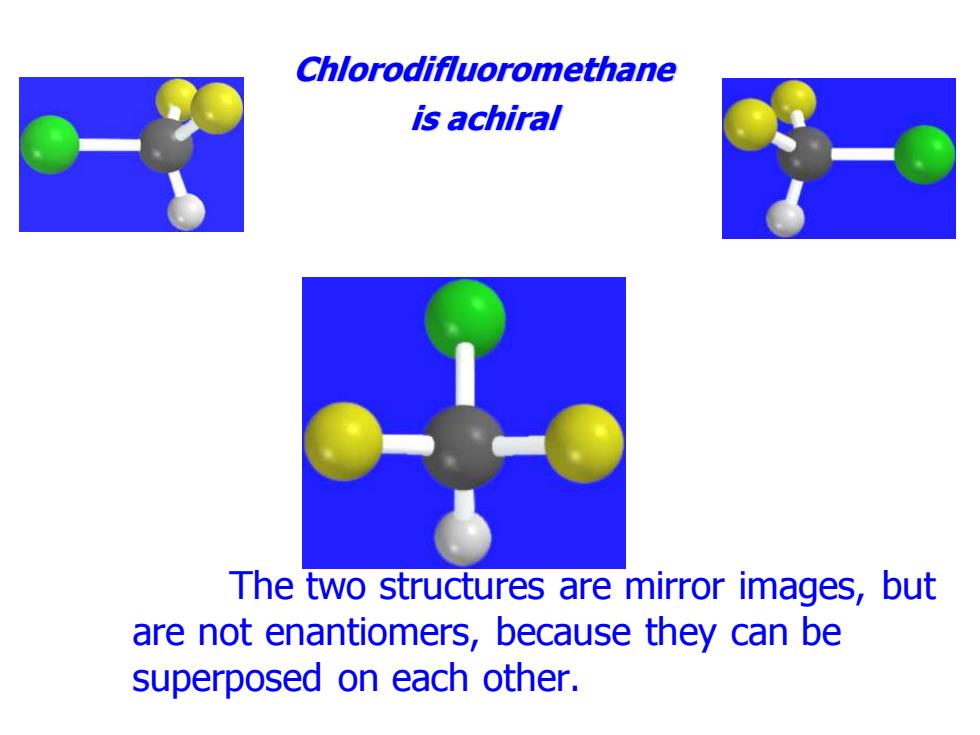
Chlorodifluoromethane is achiral The two structures are mirror images,but are not enantiomers,because they can be superposed on each other
Chlorodifluoromethane is achiral The two structures are mirror images, but are not enantiomers, because they can be superposed on each other