
有机化学ORGAnI©CHEMISTRY 主讲:主俊儒87092829(0) 理学院应化系理科接2层C206 Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 21 &T 22 Carboxylic Acids Derivatives Key Notes Hydrogen bonding;Acidity;nucleophilic substituetion claisen condensation;reduction, Homework:P732:21-18;21-19(f,g,h)P762:22-19(a-f)
Chapter 21 & 22 Carboxylic Acids & Derivatives Organic Chemistry, 6th Edition L. G. Wade, Jr. Key Notes Hydrogen bonding ; Acidity; nucleophilic substituetion; claisen condensation; reduction; 有机化学ORGANIC CHEMISTRY 主讲:王俊儒 87092829(O) 理学院应化系理科楼2层C206 Homework: P732: 21-18; 21-19(f,g,h); P762: 22-19(a-f)

Blister beetles secrete cantharidin,a powerful vesicant.Crushing a blister beetle between the fingers results in severe blistering of the skin.When horses eat hay containing blister beetles,they often die from The vinegaroon(whip-tail scorpion) gastroenteritis and kidney failure CH expels a defensive spray consisting of 84%acetic acid,5%octanoic acid. caused by cantharidin poisoning. and 11%water.Octanoic acid acts as a wetting and spreading agent. 斑蝥素 CH3O cantharidin
斑蝥素

CONTENTS ■REVIEW:NAMING STRUCTURE AND PROPERTIES NUCLEOPHILIC SUBSTITUTION ■PREPARATIONS ■REACTIONS ■ENOLATE REACTIONS
CONTENTS ◼REVIEW:NAMING ◼STRUCTURE AND PROPERTIES ◼NUCLEOPHILIC SUBSTITUTION ◼PREPARATIONS ◼REACTIONS ◼ENOLATE REACTIONS
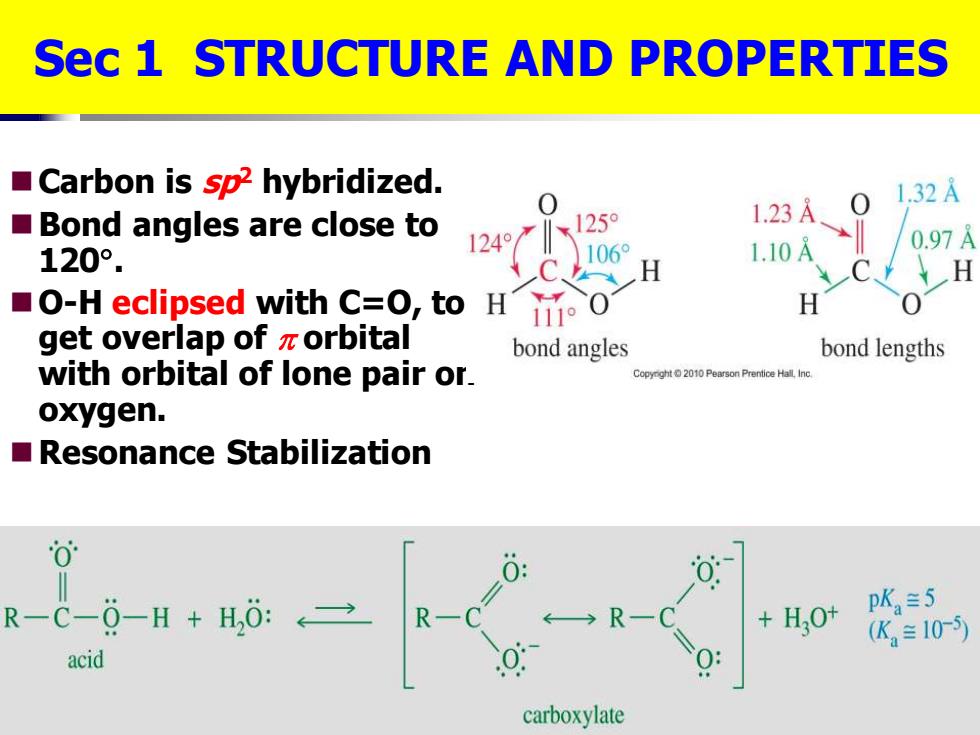
Sec 1 STRUCTURE AND PROPERTIES Carbon is sp2 hybridized. 1247X12 0 1.32A Bond angles are close to 1.23A 120°. 1060 1.10A 0.97 H O-H eclipsed with C=O,to H 111°0 H get overlap of zorbital bond angles bond lengths with orbital of lone pair or. Copyght2010 Pearson Prentice Hall Inc oxygen. Resonance Stabilization 0 R-C-0-H H,0: +H,0t pK,=5 R- (K≡10-5) acid carboxylate
◼Carbon is sp2 hybridized. ◼Bond angles are close to 120 . ◼O-H eclipsed with C=O, to get overlap of orbital with orbital of lone pair on oxygen. ◼Resonance Stabilization Sec 1 STRUCTURE AND PROPERTIES
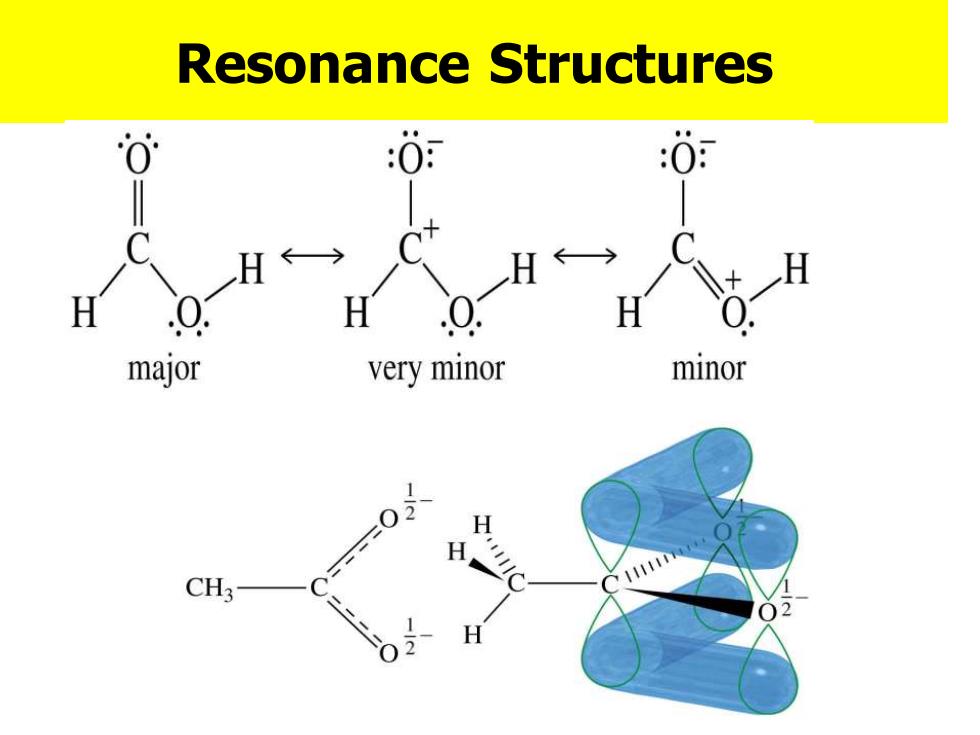
Resonance Structures :0 :0 YX H H 一H H Q major very minor minor H H CH3
Resonance Structures
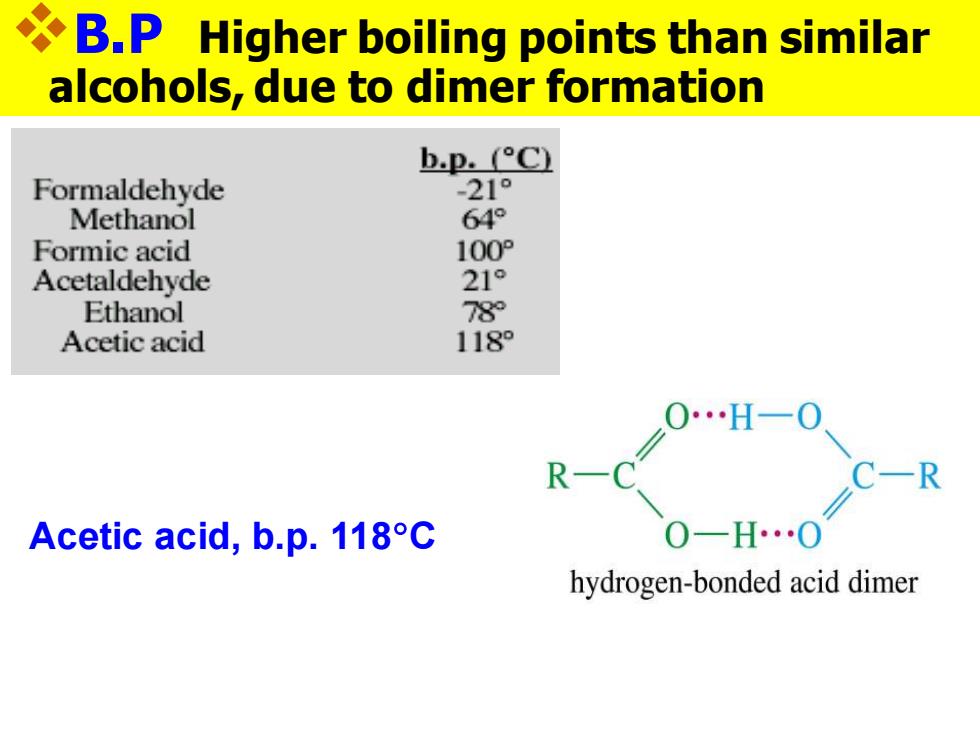
B.P Higher boiling points than similar alcohols,due to dimer formation bp.°C Formaldehyde -21° Methanol 64° Formic acid 100° Acetaldehyde 21° Ethanol 78 Acetic acid 118 0…H一0 R C-R Acetic acid,b.p.118C hydrogen-bonded acid dimer
❖B.P Higher boiling points than similar alcohols, due to dimer formation Acetic acid, b.p. 118C
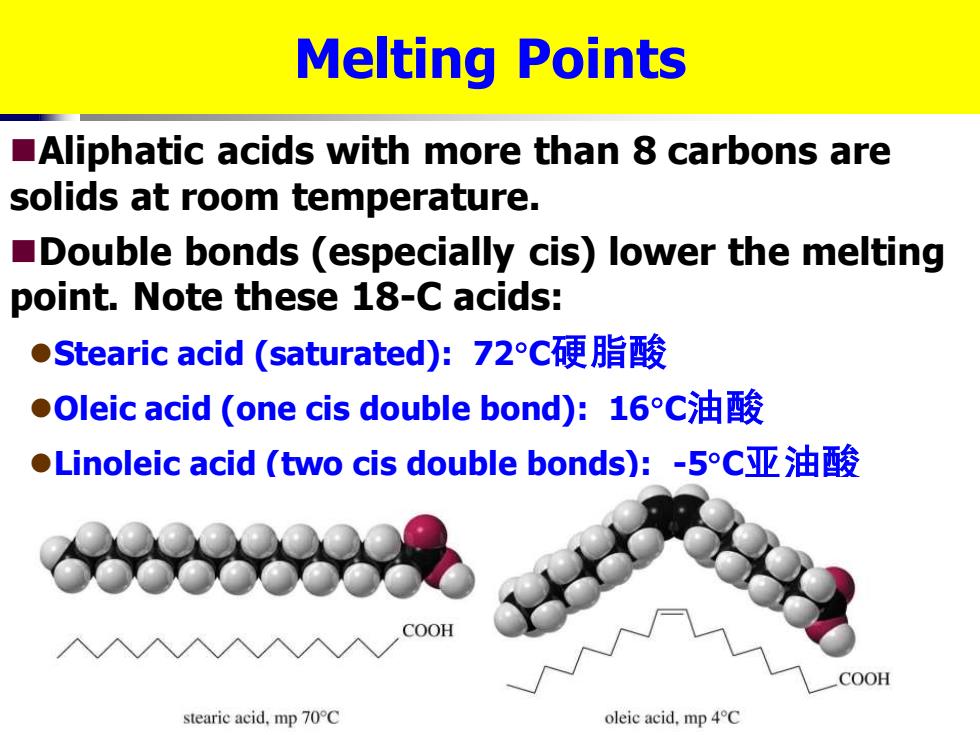
Melting Points Aliphatic acids with more than 8 carbons are solids at room temperature. Double bonds (especially cis)lower the melting point.Note these 18-C acids: ●Stearic acid(saturated):72c硬脂酸 ●Oleic acid(one cis double bond):16C油酸 ●Linoleic acid(two cis double bonds):-5c亚油酸 COOH COOH stearic acid.mp70°C oleic acid.mp 4C
Melting Points ◼Aliphatic acids with more than 8 carbons are solids at room temperature. ◼Double bonds (especially cis) lower the melting point. Note these 18-C acids: ⚫Stearic acid (saturated): 72C硬脂酸 ⚫Oleic acid (one cis double bond): 16C油酸 ⚫Linoleic acid (two cis double bonds): -5C亚油酸 硬脂酸,油酸,亚油酸

Solubility Water solubility decreases with the length of the carbon chain. Up to 4 carbons,acid is miscible in water. More soluble in alcohol. Also soluble in relatively nonpolar solvents like chloroform because it dissolves as a dimer
Solubility ◼Water solubility decreases with the length of the carbon chain. ◼Up to 4 carbons, acid is miscible in water. ◼More soluble in alcohol. ◼Also soluble in relatively nonpolar solvents like chloroform because it dissolves as a dimer
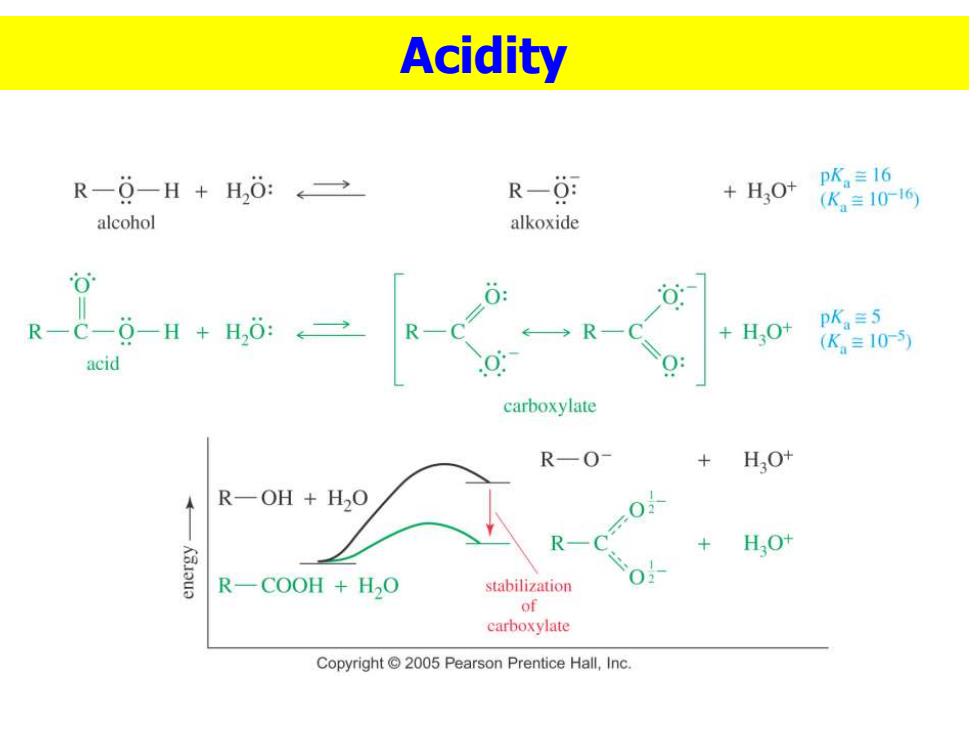
Acidity R-0-H+H,0:←→ R-0: pK。≡16 +HO+ (K。≡1016 alcohol alkoxide 0 R-C-0-H+H,:→ R HO+ pK≡5 + (K。=10-5) acid carboxylate R—O- +HO+ R-OH H2O R 0-0以 + HO+ R-COOH H2O stabilization 0 of carboxylate Copyright 2005 Pearson Prentice Hall,Inc
Acidity
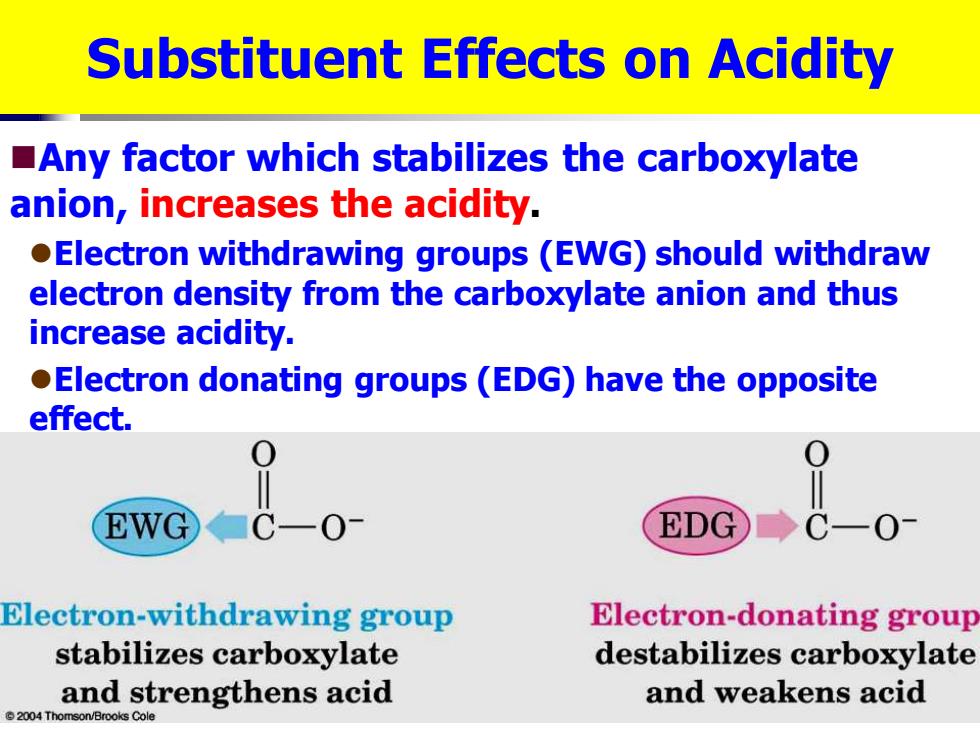
Substituent Effects on Acidity Any factor which stabilizes the carboxylate anion,increases the acidity. Electron withdrawing groups (EWG)should withdraw electron density from the carboxylate anion and thus increase acidity. Electron donating groups(EDG)have the opposite effect. EWG ■CO1 EDG C-0 Electron-withdrawing group Electron-donating group stabilizes carboxylate destabilizes carboxylate and strengthens acid and weakens acid 2004 Thomson/Brooks Cole
Substituent Effects on Acidity ◼Any factor which stabilizes the carboxylate anion, increases the acidity. ⚫Electron withdrawing groups (EWG) should withdraw electron density from the carboxylate anion and thus increase acidity. ⚫Electron donating groups (EDG) have the opposite effect