
自标转对 Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 2 Structure and Properties of OMs Key Notes VSEPRtheory;Pi Bonding;HybridOrbitals; Molecular Orbital Molecular Shapes;Isomerismj Bond and Molecular Dipole Moments;Intermolecular Forcesj Boiling Points and Solubility; By Junru Wang Email:wangjr07@163.com
By Junru Wang Email: wangjr07@163.com Chapter 2 Structure and Properties of OMs Organic Chemistry, 6th Edition L. G. Wade, Jr. Key Notes VSEPR theory; Pi Bonding; Hybrid Orbitals; Molecular Orbital; Molecular Shapes; Isomerism; Bond and Molecular Dipole Moments; Intermolecular Forces; Boiling Points and Solubility;
自秋不转大对 CONTENTS 1.Bonding Molecular Orbital 2.Hybridization Molecular Shapes Special topic:Hybridization of carbon species,N,O,P,and S 3.Isomerism 4.3D Molecules Drawing 5.Polarity of Bonds Molecules 6.Intermolecular Forces their effects Homework:2-27:2-28:2-33:2-34P64)
CONTENTS 1. Bonding & Molecular Orbital 2. Hybridization & Molecular Shapes ◼Special topic: Hybridization of carbon species,N,O,P,and S 3. Isomerism 4. 3D Molecules Drawing 5. Polarity of Bonds & Molecules 6. Intermolecular Forces & their effects Homework:2-27;2-28;2-33;2-34(P64)

SEC 1 Bonding Molecular Orbital Wave Properties of Electrons Standing wave vibrates in fixed location. ■ Wave function,w,mathematical description of size,shape,orientation. Amplitude may be positive or negative. Node:amplitude is zero. nodal plane nucleus represented by nucleus wave function (instantaneous picture) nodal plane
SEC 1 Bonding & Molecular Orbital Wave Properties of Electrons ◼ Standing wave vibrates in fixed location. ◼ Wave function, , mathematical description of size, shape, orientation. ◼ Amplitude may be positive or negative. ◼ Node: amplitude is zero
Wave Interactions LCAO:Linear combination of atomic orbitals on different atoms produce molecular orbitals oon the same atom give hybrid orbitals. Conservation of orbitals. Waves that are in phase add together. Amplitude increases. Waves that are out of phase cancel out
Wave Interactions ◼LCAO:Linear combination of atomic orbitals ⚫on different atoms produce molecular orbitals ⚫on the same atom give hybrid orbitals. ◼Conservation of orbitals. ◼Waves that are in phase add together. Amplitude increases. ◼Waves that are out of phase cancel out

自标4花对 Bonding Region ■ Electrons are close to both nuclei. bonding region ● electrons in this region nucleus 1 nucleus 2 attract both nuclei and mask the positive charges from repelling each other Copyright2005 Pearson Prentice Hall,Inc
Bonding Region ◼Electrons are close to both nuclei

自秋转达对 Sigma(o)Bonding ■ Electron density lies between the nuclei. A bond may be formed by s-s,p-p,s-p or hybridized orbital overlaps. ■ The bonding MO is lower in energy than the original atomic orbitals. The antibonding MO is higher in energy than the atomic orbitals
Sigma(σ) Bonding ◼Electron density lies between the nuclei. ◼A bond may be formed by s-s, p-p, s-p, or hybridized orbital overlaps. ◼The bonding MO is lower in energy than the original atomic orbitals. ◼The antibonding MO is higher in energy than the atomic orbitals
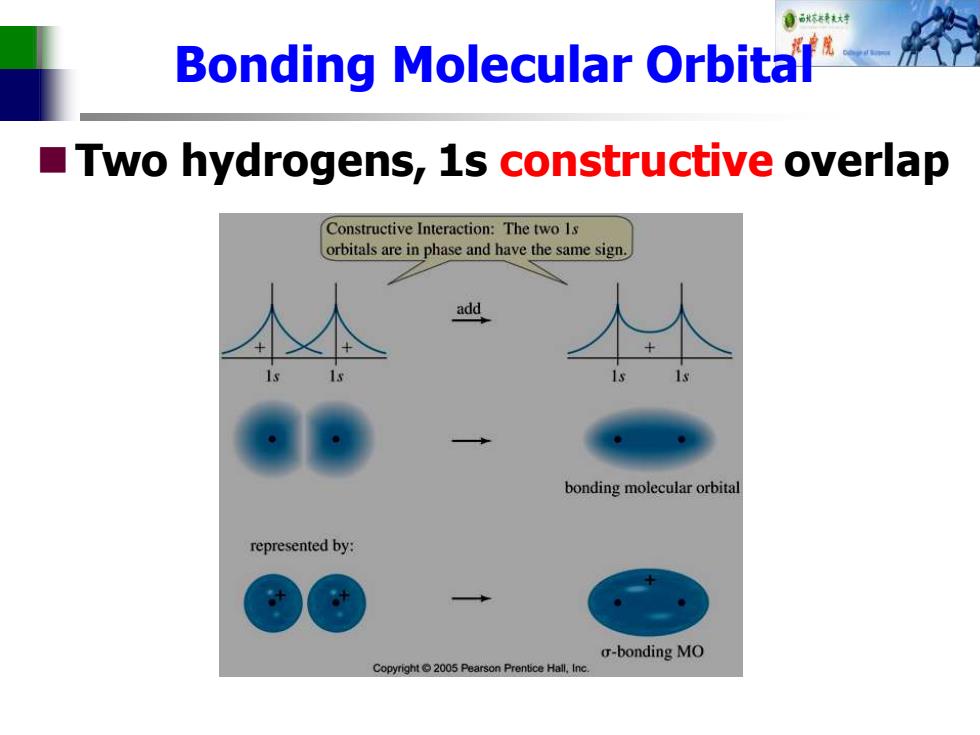
Bonding Molecular Orbital Two hydrogens,1s constructive overlap Constructive Interaction:The two 1s orbitals are in phase and have the same sign add bonding molecular orbital represented by: o-bonding MO Copyright2005 Pearson Prentice Hall,Inc
Bonding Molecular Orbital ◼Two hydrogens, 1s constructive overlap
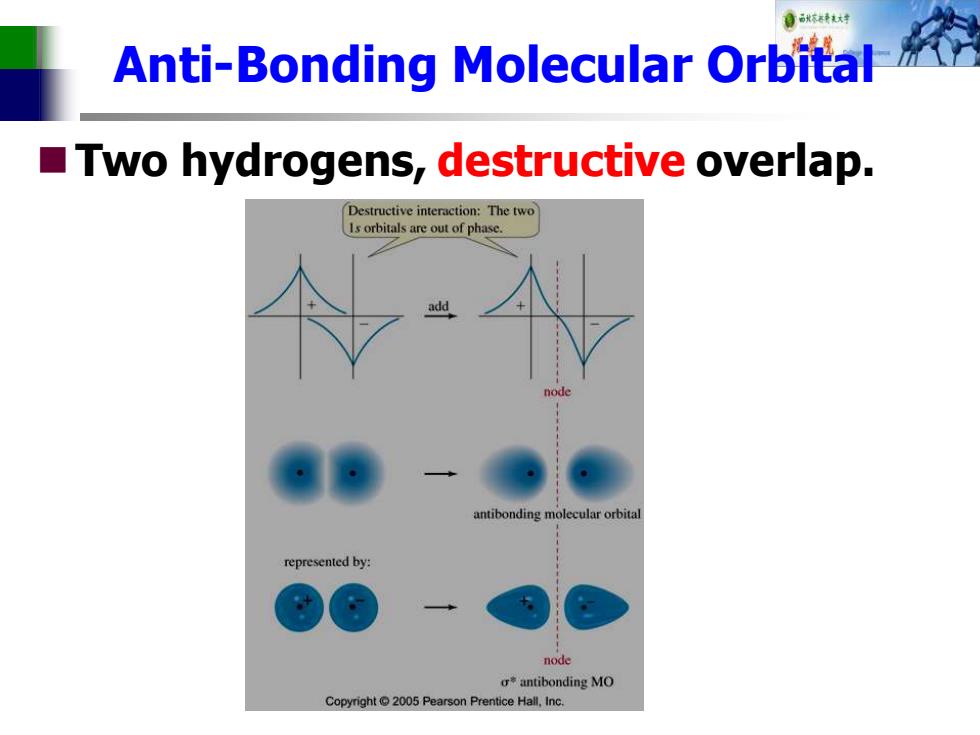
自秋转对 Anti-Bonding Molecular Orbital Two hydrogens,destructive overlap. Destructive interaction:The two Is orbitals are out of phase. add node antibonding molecular orbital represented by: node oantibonding MO Copyright 2005 Pearson Prentice Hall,Inc
Anti-Bonding Molecular Orbital ◼Two hydrogens, destructive overlap
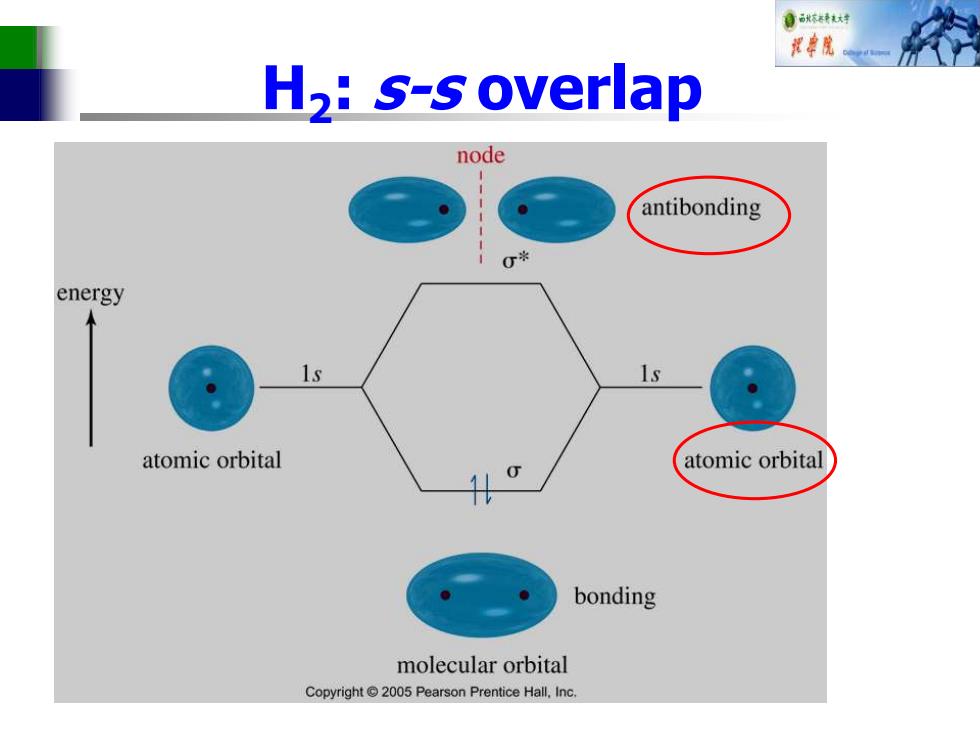
自秋转大材 花单院 H,:s-s overlap node antibonding 0* energy 1s atomic orbital atomic orbital bonding molecular orbital Copyright 2005 Pearson Prentice Hall.Inc
H2 : s-s overlap
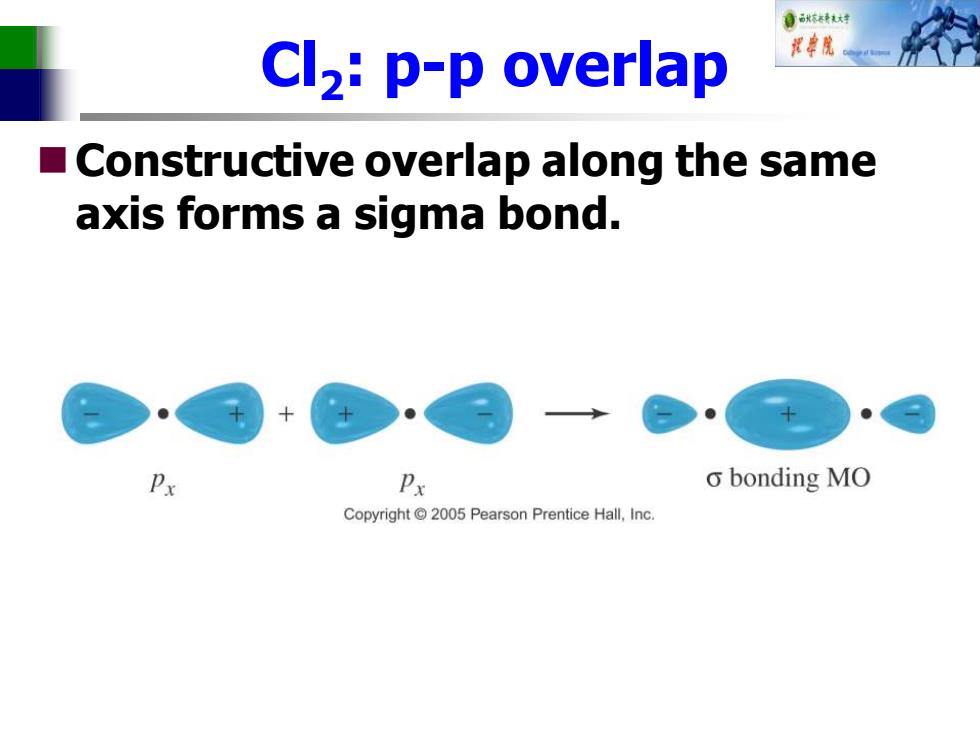
自秋不转大对 Cl2:p-p overlap ■ Constructive overlap along the same axis forms a sigma bond. Px Px o bonding MO Copyright 2005 Pearson Prentice Hall,Inc
Cl2 : p-p overlap ◼Constructive overlap along the same axis forms a sigma bond