
Organic Chemistry with Biological Applications,3r Edition John McMurry Chapter 2 Polar covalent Bonds;acids and Bases Understanding organic and biological chemistry means knowing not just what happens but also why and how it happens at the molecular level. By Junru Wang College of Chemistry and Pharmacy Room C206,Science Building Tel:15109273921;Email:wangjr07@163.com
By Junru Wang College of Chemistry and Pharmacy Room C206, Science Building Tel: 15109273921; Email: wangjr07@163.com Chapter 2 Polar covalent Bonds; acids and Bases Organic Chemistry with Biological Applications, 3rd Edition John McMurry Understanding organic and biological chemistry means knowing not just what happens but also why and how it happens at the molecular level
Organic Chemistry with Biological Applications,3r Edition John McMurry Chapter 2 Polar covalent Bonds;acids and Bases Homework:2-2 (P31),2-6, 2-22P58d)2-26,2-34,2-38 Key Notes Electronegativity,Dipole Moments,Formal Charges Resonance,Acids and Bases
Chapter 2 Polar covalent Bonds; acids and Bases Organic Chemistry with Biological Applications, 3rd Edition John McMurry Key Notes Electronegativity , Dipole Moments, Formal Charges Resonance, Acids and Bases Homework: 2-2 (P31),2-6, 2-22(P58d), 2-26,2-34,2-38
Main Contents Polarity of Bonds Molecules √Electronegativity √Dipole Moments ☐Formal Charges ResonanceResonance Forms ■Acids and Bases The Bronsted-Lowry Definition √The Lewis Definition Noncovalent Interactions Between Molecules
Main Contents Polarity of Bonds & Molecules Electronegativity Dipole Moments Formal Charges Resonance & Resonance Forms Acids and Bases The Brønsted–Lowry Definition The Lewis Definition Noncovalent Interactions Between Molecules
Sec 1 Polarity of Bonds Molecules Chemical bonds lonic bonds Bond in sodium chloride is ionic -Sodium transfers an electron to chlorine to give Na*and CI lons held together by electrostatic attractions between unlike charges Nonpolar Covalent bonds Two electrons are shared equally by the two bonding atoms Carbon-carbon bond in ethane -Symmetrical electron distribution in the bond Most bonds are neither fully ionic or covalent
Chemical bonds Ionic bonds Bond in sodium chloride is ionic Sodium transfers an electron to chlorine to give Na+ and Cl- Ions held together by electrostatic attractions between unlike charges Nonpolar Covalent bonds Two electrons are shared equally by the two bonding atoms Carbon-carbon bond in ethane Symmetrical electron distribution in the bond Most bonds are neither fully ionic or covalent ! Sec 1 Polarity of Bonds & Molecules
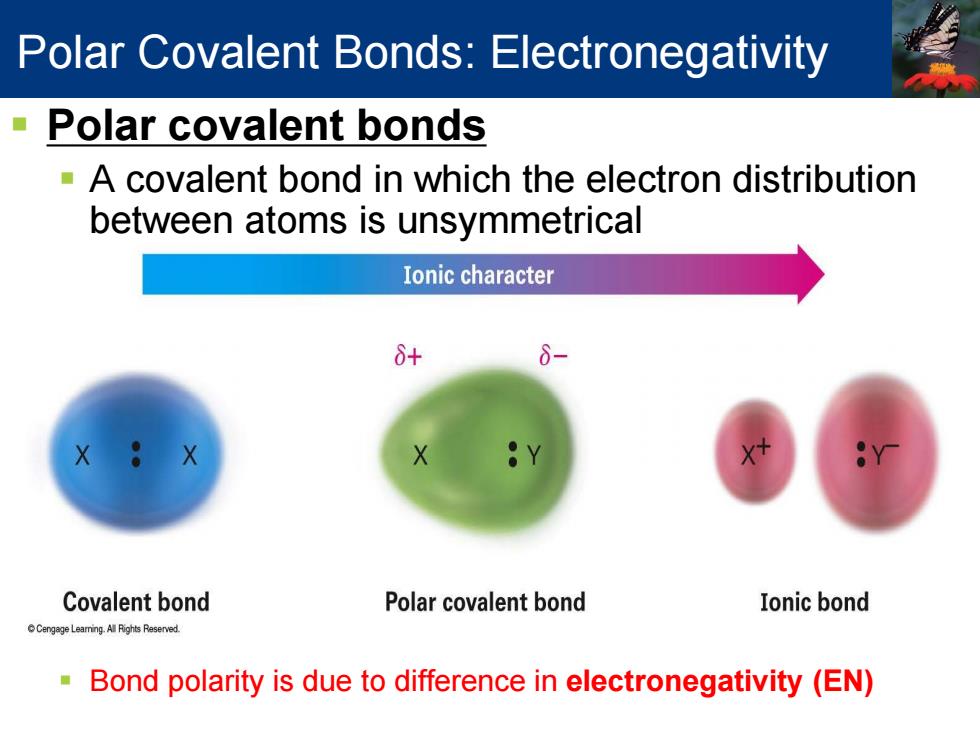
Polar Covalent Bonds:Electronegativity Polar covalent bonds -A covalent bond in which the electron distribution between atoms is unsymmetrical Ionic character 8+ Covalent bond Polar covalent bond Ionic bond Cengage Leaming.Al Rights Reserved. Bond polarity is due to difference in electronegativity (EN)
Polar covalent bonds A covalent bond in which the electron distribution between atoms is unsymmetrical Bond polarity is due to difference in electronegativity (EN) Polar Covalent Bonds: Electronegativity
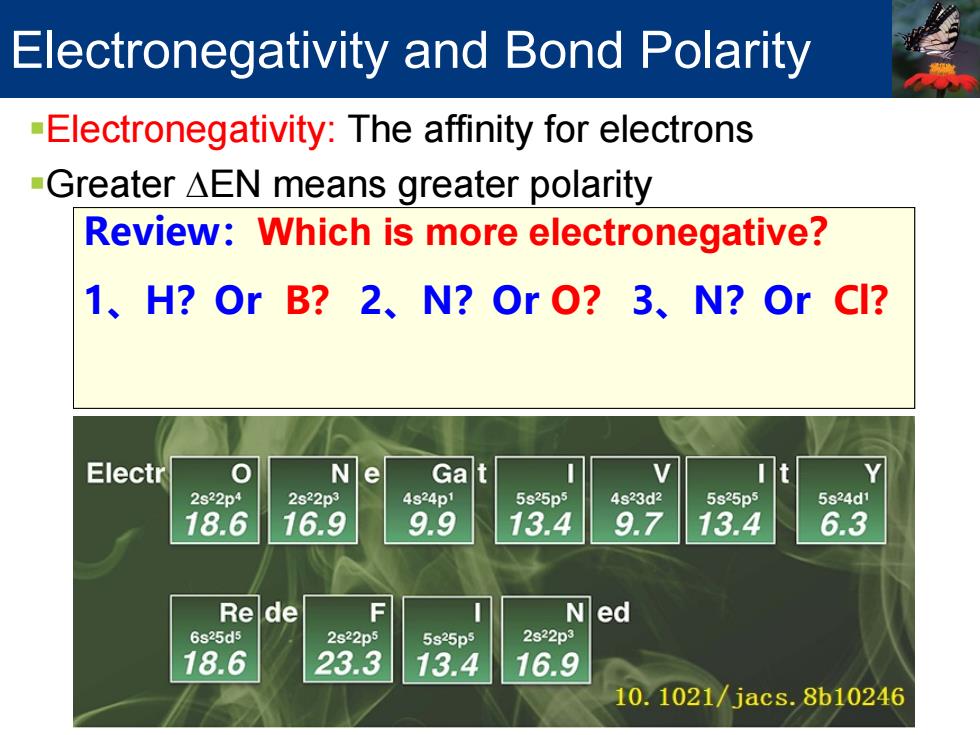
Electronegativity and Bond Polarity -Electronegativity:The affinity for electrons -Greater AEN means greater polarity Review:Which is more electronegative? 1、H?OrB?2、N?OrO?3、N?OrCI? Electr 0 Ne Gat 2s22p4 2s22p3 4s24p1 5s25p5 4s23d2 5s25p5 5s24d1 18.6 16.9 9.9 13.4 9.7 13.4 6.3 Rede F Ned 6s25d5 2s22p5 5s25p5 2s22p3 18.6 23.3 13.4 16.9 10.1021/jacs.8b10246
Electronegativity: H: 2.1 B: 2.0 C: 2.5 N: 3.0 O: 3.5 F: 4.0 Cl: 3.0 Br: 2.8 I: 2.5 Li: 1.0 Mg: 1.2 Review:Which is more electronegative? 1、H?Or B? 2、N?Or O? 3、N?Or Cl? Electronegativity and Bond Polarity Electronegativity: The affinity for electrons Greater ∆EN means greater polarity

Electronegativity and Bond Polarity 电负性 元素周期表第三维度,衡量不同原子吸引电子的强度; 定义 分子中原子吸引电子的能力(19世纪瑞典永斯~贝采利鸟斯Jòns Jacob B erzelius及美国莱纳斯•鲍林Linus Pauling) √电子亲合性和第一电离势的平均值(同期,诺贝尔化学奖得主罗伯特·马 利肯Robert Mulliken) √电负性是费米能量的负值(化学家Robert Parr) ·问题 √标度只对周期表中部分元素适用;预言化学反应或键极性时偶尔失效。 新定义和标度 √价电子平均构型能(M.Rahm副教授2019.2;诺奖得主R.Hoffmann19981) https://pubs.acs.org.ccindex.cn/doi/pdf/10.1021/jacs.8b10246
电负性 元素周期表第三维度,衡量不同原子吸引电子的强度; 定义 分子中原子吸引电子的能力(19世纪瑞典永斯•贝采利乌斯Jöns Jacob B erzelius及美国莱纳斯•鲍林Linus Pauling) 电子亲合性和第一电离势的平均值(同期,诺贝尔化学奖得主罗伯特•马 利肯Robert Mulliken) 电负性是费米能量的负值(化学家Robert Parr) 问题 标度只对周期表中部分元素适用;预言化学反应或键极性时偶尔失效。 新定义和标度 价电子平均构型能(M.Rahm副教授2019.2;诺奖得主R. Hoffmann1981) https://pubs.acs.org.ccindex.cn/doi/pdf/10.1021/jacs.8b10246 Electronegativity and Bond Polarity
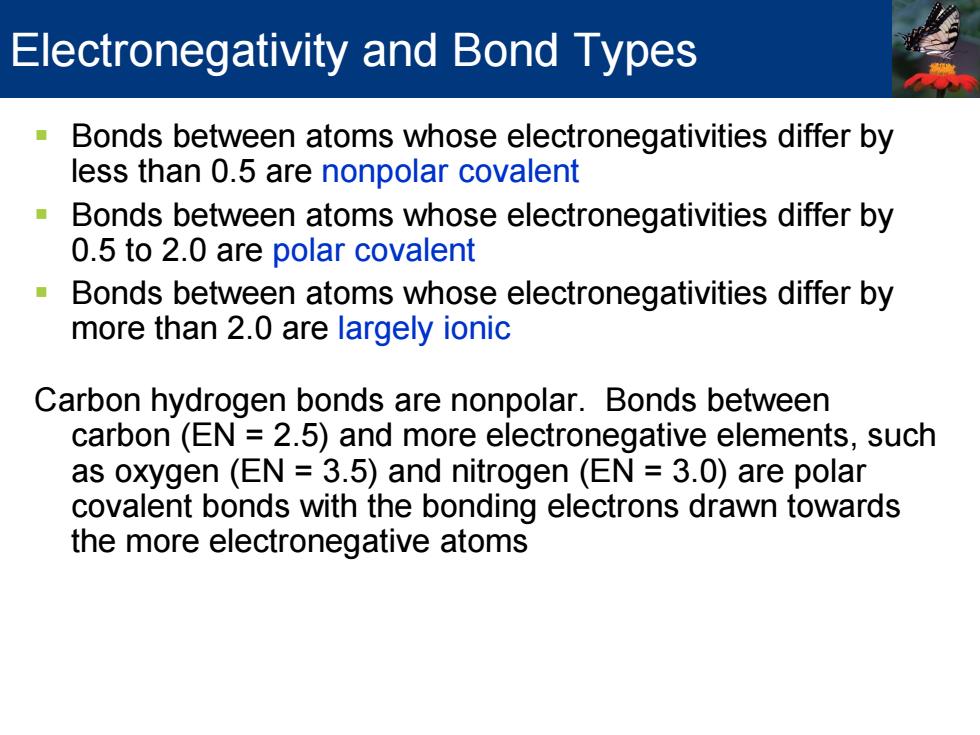
Electronegativity and Bond Types Bonds between atoms whose electronegativities differ by less than 0.5 are nonpolar covalent Bonds between atoms whose electronegativities differ by 0.5 to 2.0 are polar covalent Bonds between atoms whose electronegativities differ by more than 2.0 are largely ionic Carbon hydrogen bonds are nonpolar.Bonds between carbon(EN 2.5)and more electronegative elements,such as oxygen(EN 3.5)and nitrogen (EN 3.0)are polar covalent bonds with the bonding electrons drawn towards the more electronegative atoms
Bonds between atoms whose electronegativities differ by less than 0.5 are nonpolar covalent Bonds between atoms whose electronegativities differ by 0.5 to 2.0 are polar covalent Bonds between atoms whose electronegativities differ by more than 2.0 are largely ionic Carbon hydrogen bonds are nonpolar. Bonds between carbon (EN = 2.5) and more electronegative elements, such as oxygen (EN = 3.5) and nitrogen (EN = 3.0) are polar covalent bonds with the bonding electrons drawn towards the more electronegative atoms Electronegativity and Bond Types
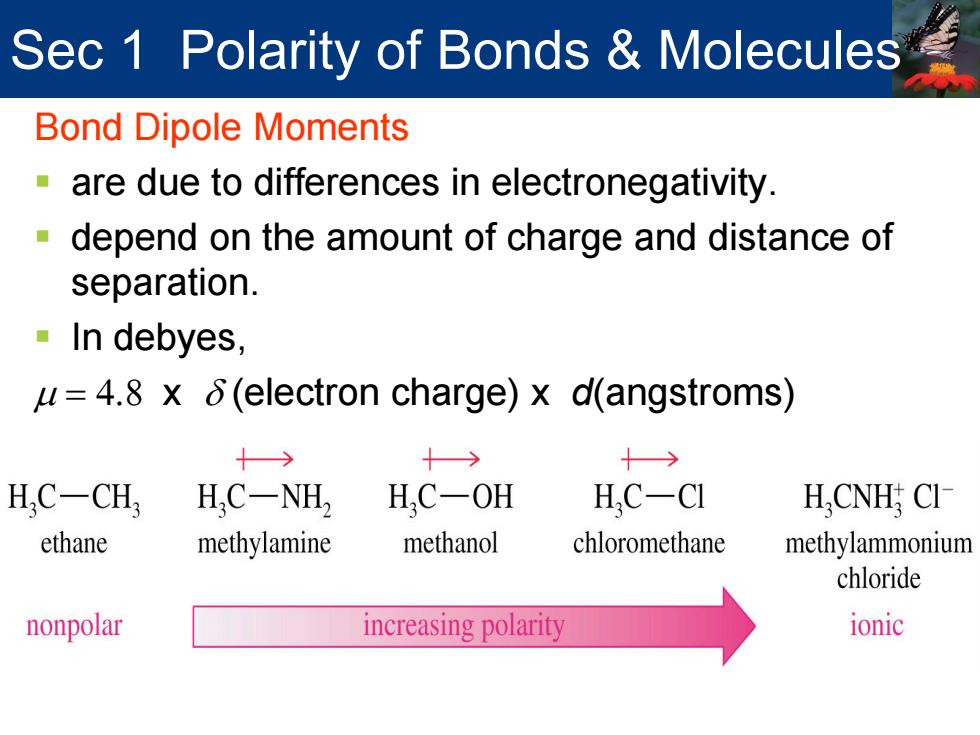
Sec 1 Polarity of Bonds Molecules Bond Dipole Moments are due to differences in electronegativity. -depend on the amount of charge and distance of separation. In debyes, u=4.8 x 6(electron charge)x d(angstroms) 十→ 十→ H.C-CH, H,C-NH2 H,C-OH H.C-CI H,CNH CI ethane methylamine methanol chloromethane methylammonium chloride nonpolar increasing polarity ionic
Sec 1 Polarity of Bonds & Molecules Bond Dipole Moments are due to differences in electronegativity. depend on the amount of charge and distance of separation. In debyes, µ = 4.8 x δ (electron charge) x d(angstroms)
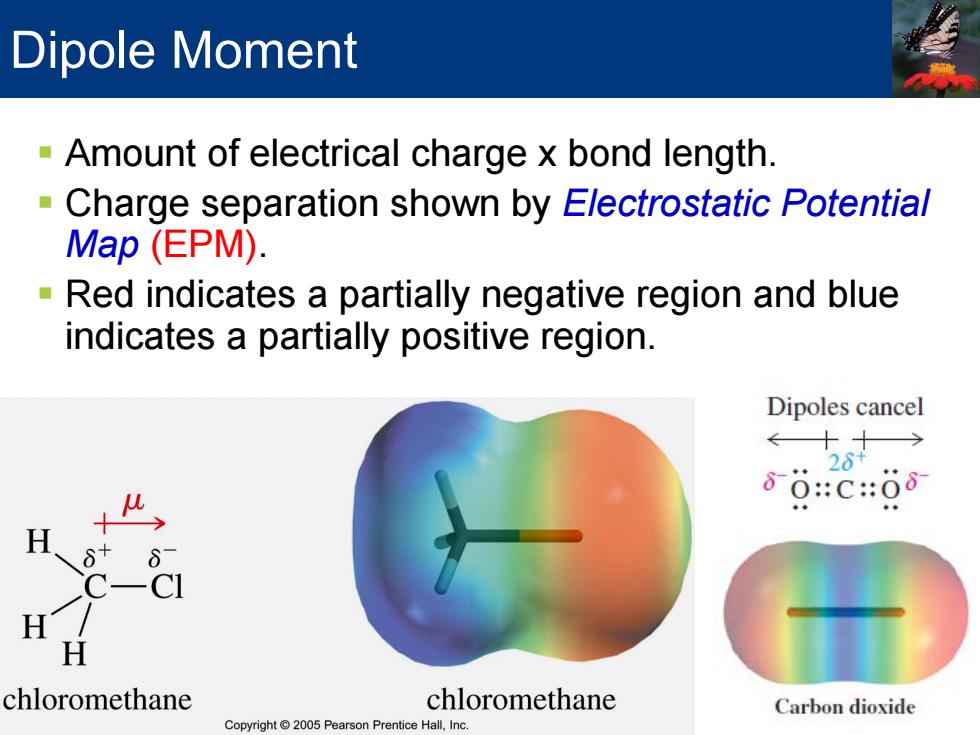
Dipole Moment Amount of electrical charge x bond length. -Charge separation shown by Electrostatic Potential Map (EPM). Red indicates a partially negative region and blue indicates a partially positive region. Dipoles cancel ←十十→ 26+ 80:c:08 H H chlo omethane chloromethane Carbon dioxide Copyright 2005 Pearson Prentice Hall,Inc
Dipole Moment Amount of electrical charge x bond length. Charge separation shown by Electrostatic Potential Map (EPM). Red indicates a partially negative region and blue indicates a partially positive region