
Organic Chemistry with Biological Applications,3 Edition John McMurry Chapter 02-SP Brief Introduction and Nomenclature of OCs Key Notes Functional group;Classification;IUPAC Names How to? Main Functional Group?Number?Configuration? Homework(to be continued): P77:3.11c-d;P90:4.1b,c,e:4.4:P185:7.4a,e,g:7.6d,e P194:7.15:P211:7.29d,e,7.31b,e
Chapter 02-SP Brief Introduction and Nomenclature of OCs Key Notes Functional group; Classification; IUPAC Names How to? Main Functional Group? Number?Configuration? Organic Chemistry with Biological Applications, 3rd Edition John McMurry Homework(to be continued): P77:3.11c-d; P90:4.1 b,c,e; 4.4; P185: 7.4a,e,g; 7.6d,e P194: 7.15; P211: 7.29d,e; 7.31b,e;

CONTENT 有机学 Classification Functional groups of OCs. Nomenclature of hydrocarbons Alkanes,Alkenes,Alkynes Aromatic hydrocarbons Nomenclature of OCs containing oxygen ●Alcohols,Ehters Aldehydes and ketones .Carboxylic acids,Carboxylic acid derivatives Nomenclature of OCs containing nitrogen Amines,Nitriles,Acid anhlides acid anhydrides Nomenclature of Multifunctional compounds
CONTENT Classification & Functional groups of OCs. Nomenclature of hydrocarbons Alkanes,Alkenes,Alkynes Aromatic hydrocarbons Nomenclature of OCs containing oxygen Alcohols,Ehters Aldehydes and ketones Carboxylic acids,Carboxylic acid derivatives Nomenclature of OCs containing nitrogen Amines,Nitriles,Acid anhlides & acid anhydrides Nomenclature of Multifunctional compounds
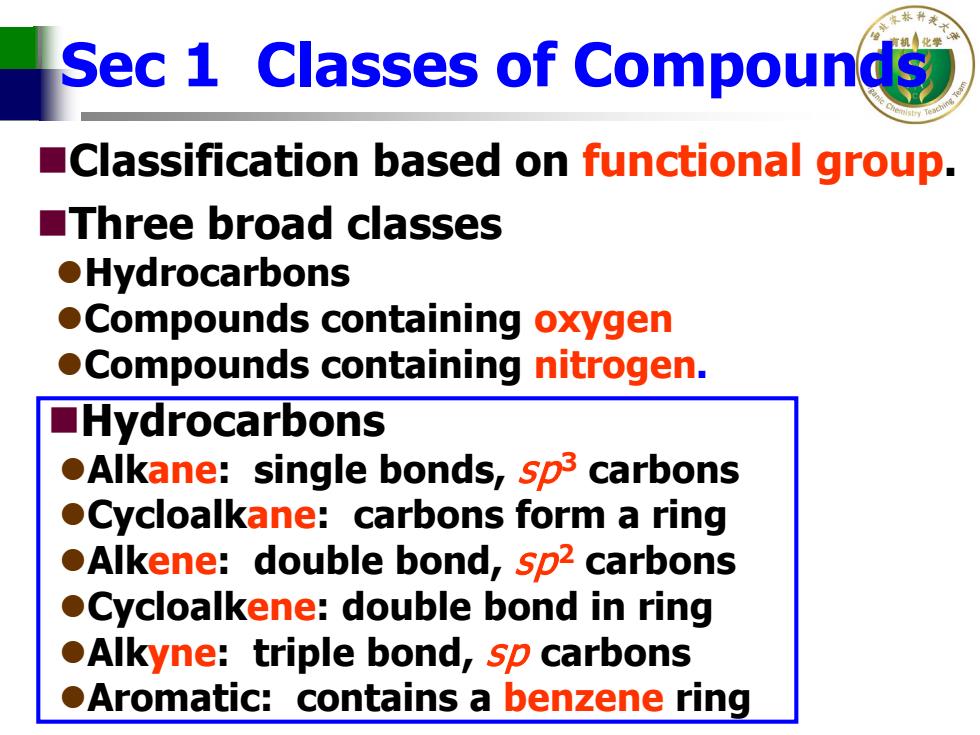
Sec 1 Classes of Compounds Classification based on functional group. ■Three broad classes ●Hydrocarbons Compounds containing oxygen Compounds containing nitrogen. ■Hydrocarbons Alkane:single bonds,sp3 carbons Cycloalkane:carbons form a ring Alkene:double bond,sp2 carbons Cycloalkene:double bond in ring Alkyne:triple bond,sp carbons .Aromatic:contains a benzene ring
Sec 1 Classes of Compounds Classification based on functional group. Three broad classes Hydrocarbons Compounds containing oxygen Compounds containing nitrogen. Hydrocarbons Alkane: single bonds, sp3 carbons Cycloalkane: carbons form a ring Alkene: double bond, sp2 carbons Cycloalkene: double bond in ring Alkyne: triple bond, sp carbons Aromatic: contains a benzene ring
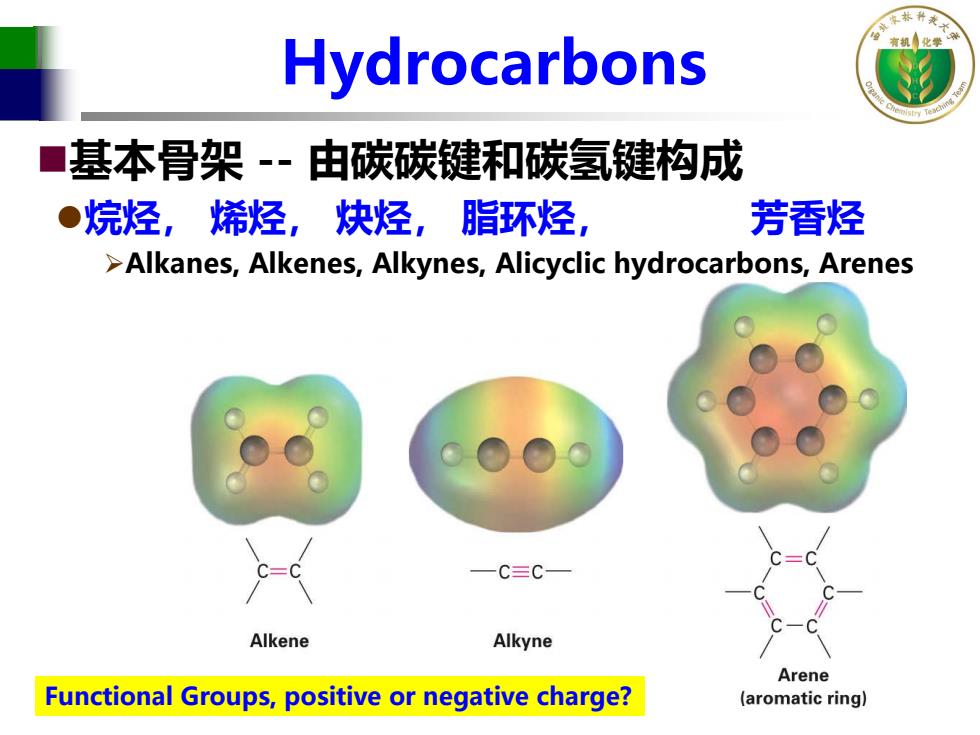
Hydrocarbons 基本骨架-由碳碳键和碳氢键构成 ●烷烃,烯烃,炔烃,脂环烃, 芳香烃 >Alkanes,Alkenes,Alkynes,Alicyclic hydrocarbons,Arenes 一C三C一 Alkene Alkyne Arene Functional Groups,positive or negative charge? (aromatic ring)
Hydrocarbons 基本骨架 -- 由碳碳键和碳氢键构成 烷烃, 烯烃, 炔烃, 脂环烃, 芳香烃 Alkanes, Alkenes, Alkynes, Alicyclic hydrocarbons, Arenes Functional Groups, positive or negative charge?
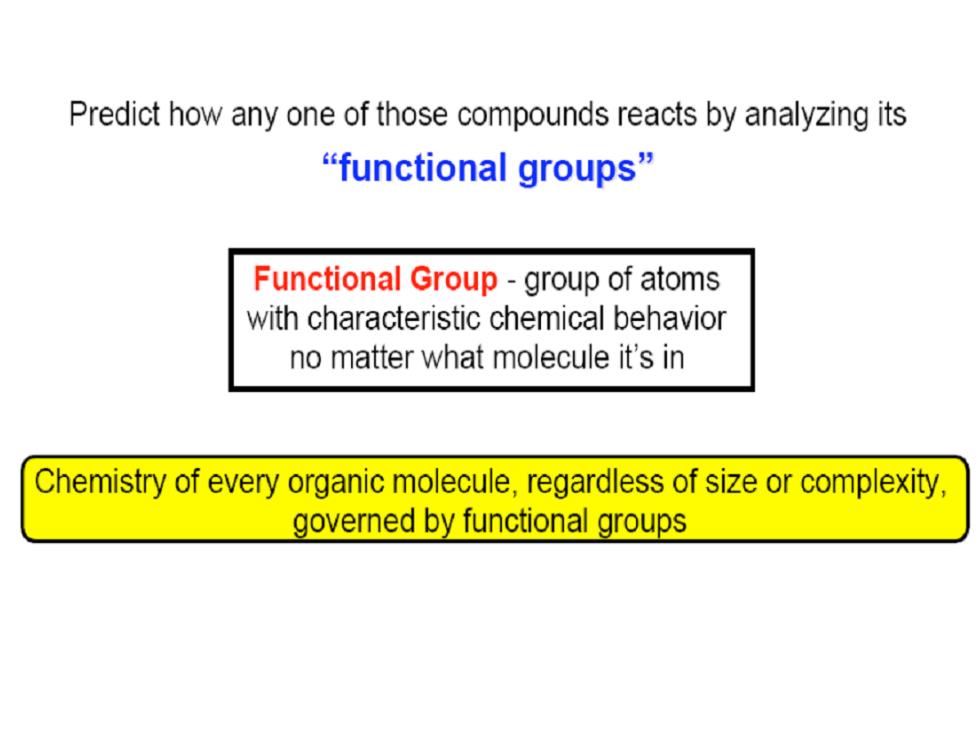
Predict how any one of those compounds reacts by analyzing its “functional groups” Functional Group-group of atoms with characteristic chemical behavior no matter what molecule it's in Chemistry of every organic molecule,regardless of size or complexity, governed by functional groups

Functional Groups ■官能团(Functional Groups): The atom or group of atoms that defines the structure of a particular family of organic compounds and,at the same time determines their properties Structural features that allow for classification of compounds into families The structural similarities in these compounds lead to chemical similarities ·官能团:有机分子中比较活泼、易发生化学反应的 原子或原子团 ●官能团:赋予有机物一类特征结构并决定其性质的 原子或原子团
Functional Groups 官能团(Functional Groups): The atom or group of atoms that defines the structure of a particular family of organic compounds and, at the same time determines their properties Structural features that allow for classification of compounds into families The structural similarities in these compounds lead to chemical similarities 官能团: 有机分子中比较活泼、易发生化学反应的 原子或原子团 官能团: 赋予有机物一类特征结构并决定其性质的 原子或原子团

Functional Groups It should be noted that the importance of a functional group cannot be overstated. A functional group affects or determines all of the following properties of a molecule: ●Bonding and shape ● Chemical reactivity Type and strength of intermolecular forces Physical properties Nomenclature
Functional Groups It should be noted that the importance of a functional group cannot be overstated. A functional group affects or determines all of the following properties of a molecule: Bonding and shape Chemical reactivity Type and strength of intermolecular forces Physical properties Nomenclature
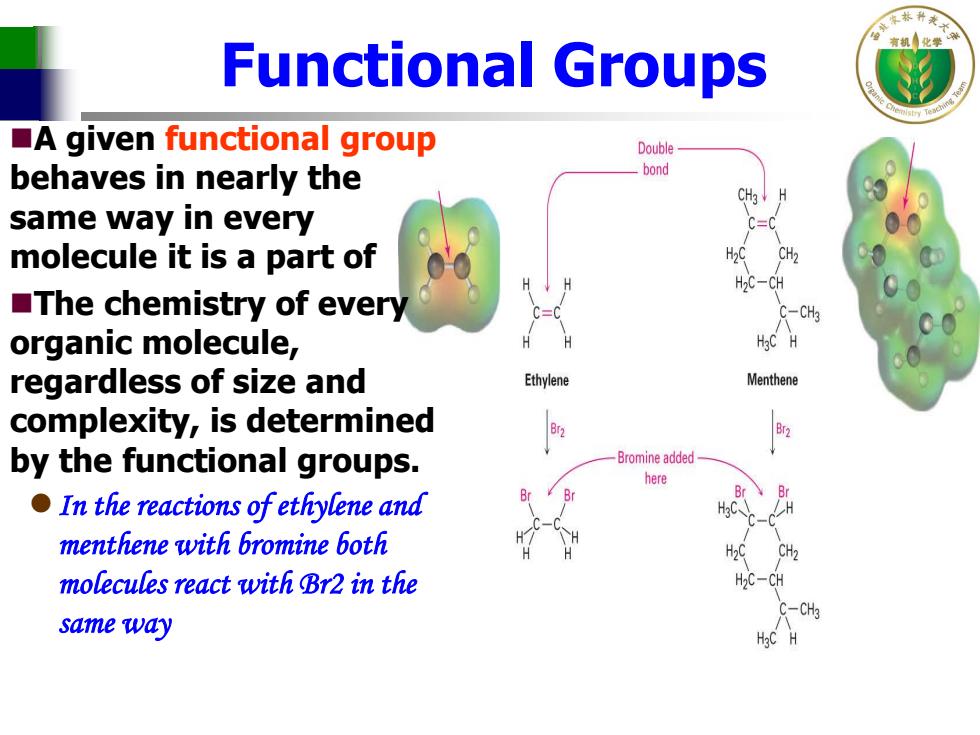
Functional Groups A given functional group Double behaves in nearly the bond CH3 same way in every =0 molecule it is a part of H2C-CH The chemistry of every C-CHa organic molecule, regardless of size and Ethylene Menthene complexity,is determined by the functional groups. -Bromine added here In the reactions ofethylene and menthene with bromine both molecules react with Br2 in the C-CH3 same way
Functional Groups A given functional group behaves in nearly the same way in every molecule it is a part of The chemistry of every organic molecule, regardless of size and complexity, is determined by the functional groups. In the reactions of ethylene and menthene with bromine both molecules react with Br2 in the same way
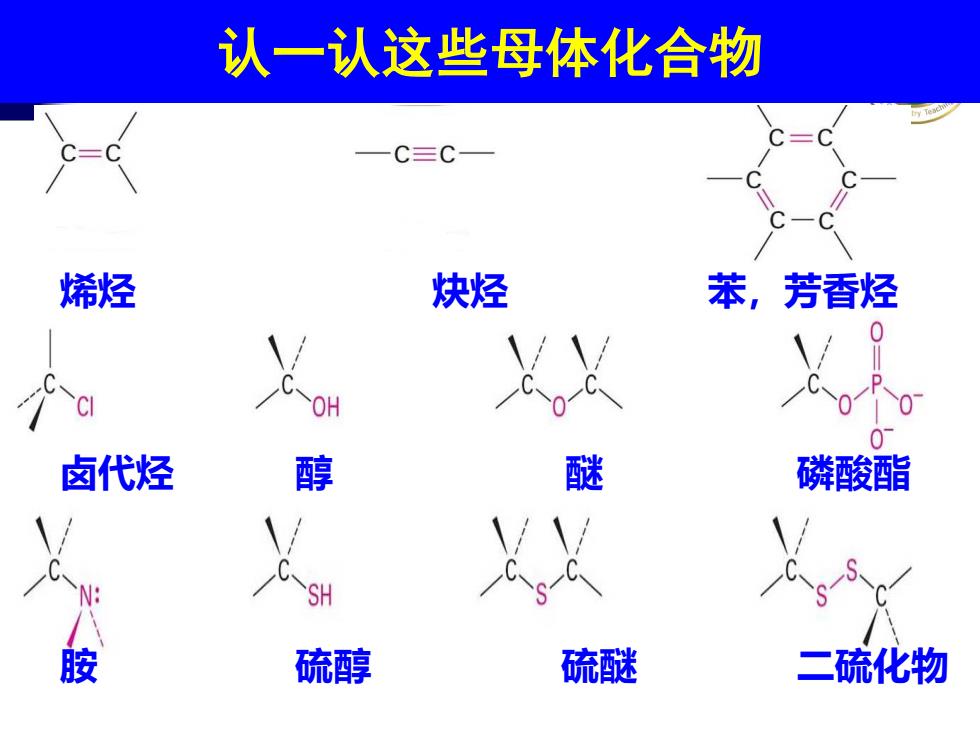
认一认这些母体化合物 C三C 烯烃 炔烃 苯,芳香烃 OH 卤代烃 醇 醚 磷酸酯 胺 硫醇 硫醚 二硫化物
认一认这些母体化合物 烯烃 卤代烃 醇 炔烃 苯,芳香烃 胺 硫醇 醚 磷酸酯 硫醚 二硫化物
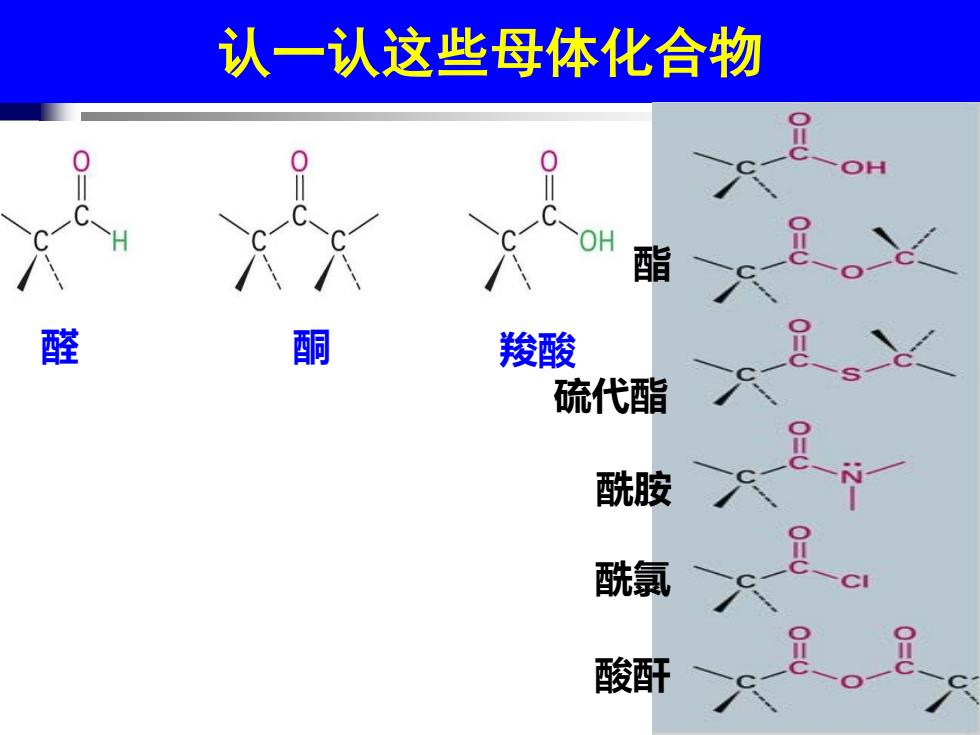
认一认这些母体化合物 OH 醛 酮 羧酸 硫代酯 酰胺 酰氯 酸酐
认一认这些母体化合物 醛 酮 羧酸 硫代酯 酯 酰胺 酰氯 酸酐