有机北学 Organic Chemistry with BiologicalApplications.Edition John McMurry Chapter 07 eT 08 alkenes and alkynes Carbon-carbon double bonds are present in most organic and biological molecules,so a good understanding of the electrophilic addition reaction is needed. Key Notes Electrophile;Electrophilic addition; Carbocation stability;Conjugated dienes
Chapter 07 & 08 alkenes and alkynes Organic Chemistry with Biological Applications, 3rd Edition John McMurry Carbon–carbon double bonds are present in most organic and biological molecules, so a good understanding of the electrophilic addition reaction is needed. Key Notes Electrophile; Electrophilic addition; Carbocation stability; Conjugated dienes

有机化学网 Organic Chemistry with Biological Applications,3 Edition John McMurry Chapter 07 08 alkenes and alkynes Homework:P203:7.18,:7.21:P211e:7.47;7.50:7.51 P231:8.12a,P264c:8.34:8.36;8.47acd:8.60abcd By Junru Wang College of Chemistry and Pharmacy Room C206,Science Building .m i071
By Junru Wang College of Chemistry and Pharmacy Room C206, Science Building Tel: 87092829(O);Email: wangjr07@163 com Chapter 07 & 08 alkenes and alkynes Organic Chemistry with Biological Applications, 3rd Edition John McMurry Homework: P203:7.18; 7.21; P211e: 7.47; 7.50; 7.51; P231:8.12a; P264c:8.34 ;8.36; 8.47acd; 8.60abcd;

萜烯--天然存在的烯烃Terpenoids 公元1000年,波斯 ■水汽蒸馏-一植物一挥发油(精油) ·药物,香料,香水等 ■19世纪:-促进有机化学作为一门科学出现; ■i 萜烯类化合物 ·超过35000种天然萜烯类; ·骨架多样性--单萜,倍半萜,二萜,三萜,多萜 ■部位各异:玫瑰的花、薄荷的叶、檀香树干、桂树 皮、当归根、茴香果实、白豆蔻种子 ■功能活性有20多种:
萜烯---天然存在的烯烃Terpenoids 公元1000年,波斯 水汽蒸馏---植物—挥发油(精油) 药物,香料,香水等 19世纪:--促进有机化学作为一门科学出现; 萜烯类化合物 超过35000种天然萜烯类; 骨架多样性---单萜,倍半萜,二萜,三萜,多萜 部位各异:玫瑰的花、薄荷的叶、檀香树干、桂树 皮、当归根、茴香果实、白豆蔻种子 功能活性有20多种:

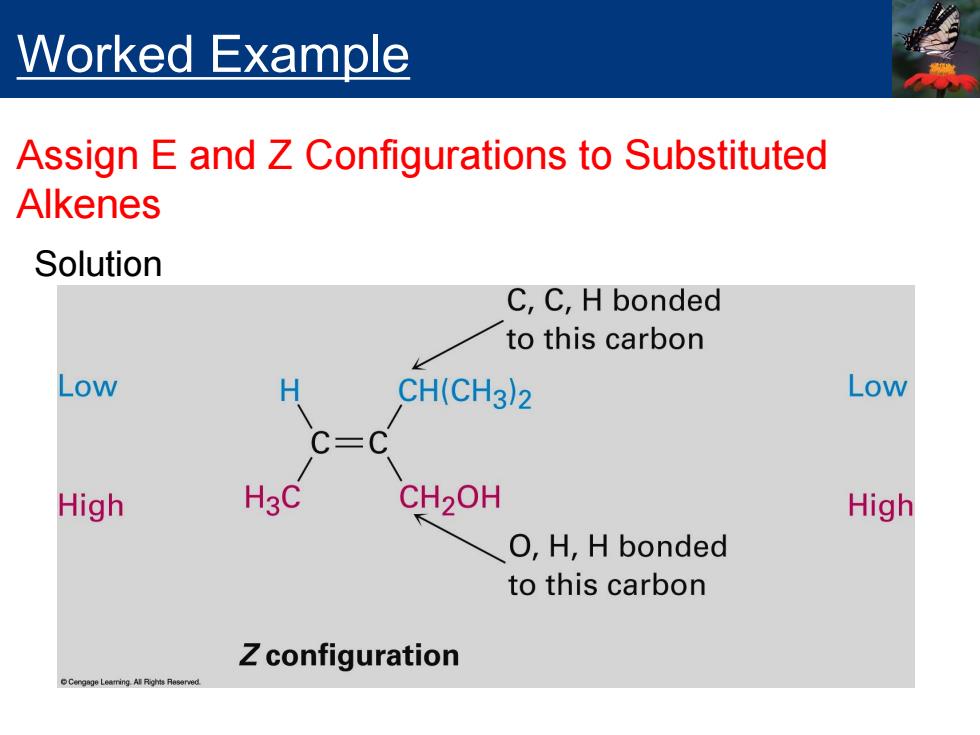
Worked Example Assign E and Z Configurations to Substituted Alkenes Solution C,C,H bonded to this carbon Low H CH(CH3)2 Low C=C High H3C CH2OH High O,H,H bonded to this carbon Z configuration
Solution Worked Example Assign E and Z Configurations to Substituted Alkenes

Main Contents Stability of alkenes Preparing alkenes:a preview EA Reactions of alkenes Carbocation structure and stability Reduction oxidation of alkenes Biological additions of Radicals to alkenes ■Conjugated Dienes ■Reactions of alkynes
Main Contents Stability of alkenes Preparing alkenes: a preview EA Reactions of alkenes Carbocation structure and stability Reduction & oxidation of alkenes Biological additions of Radicals to alkenes Conjugated Dienes Reactions of alkynes
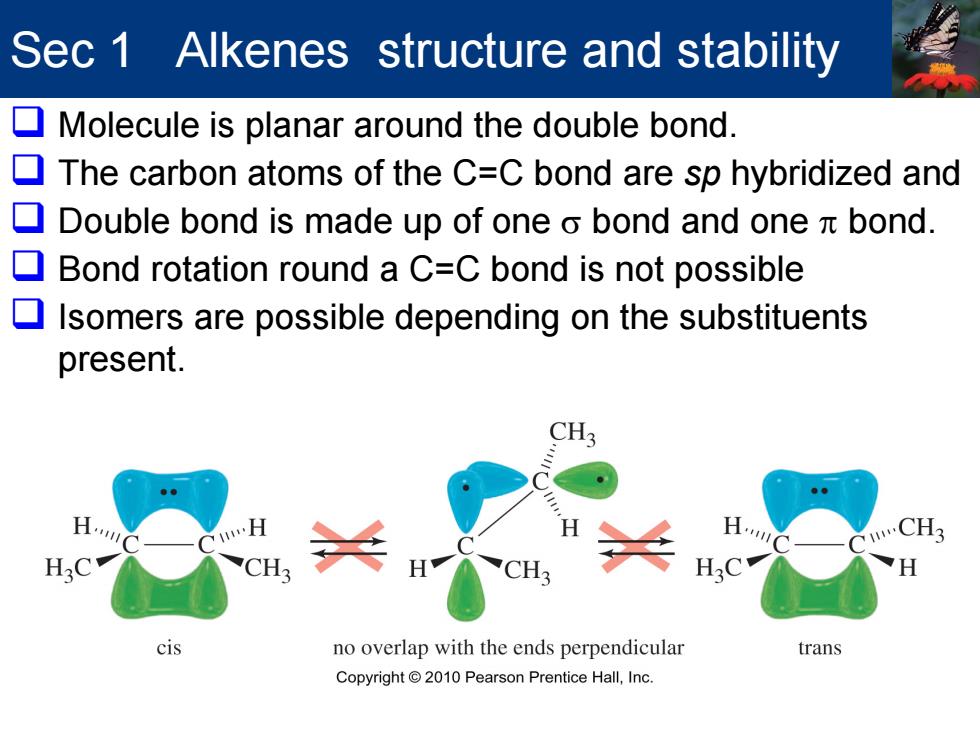
Sec 1 Alkenes structure and stability Molecule is planar around the double bond. 口 The carbon atoms of the C=C bond are sp hybridized and Double bond is made up of one o bond and one bond. Bond rotation round a C=C bond is not possible Isomers are possible depending on the substituents present. CH3 CH3 cis no overlap with the ends perpendicular trans Copyright 2010 Pearson Prentice Hall,Inc
Sec 1 Alkenes structure and stability Molecule is planar around the double bond. The carbon atoms of the C=C bond are sp hybridized and Double bond is made up of one σ bond and one π bond. Bond rotation round a C=C bond is not possible Isomers are possible depending on the substituents present
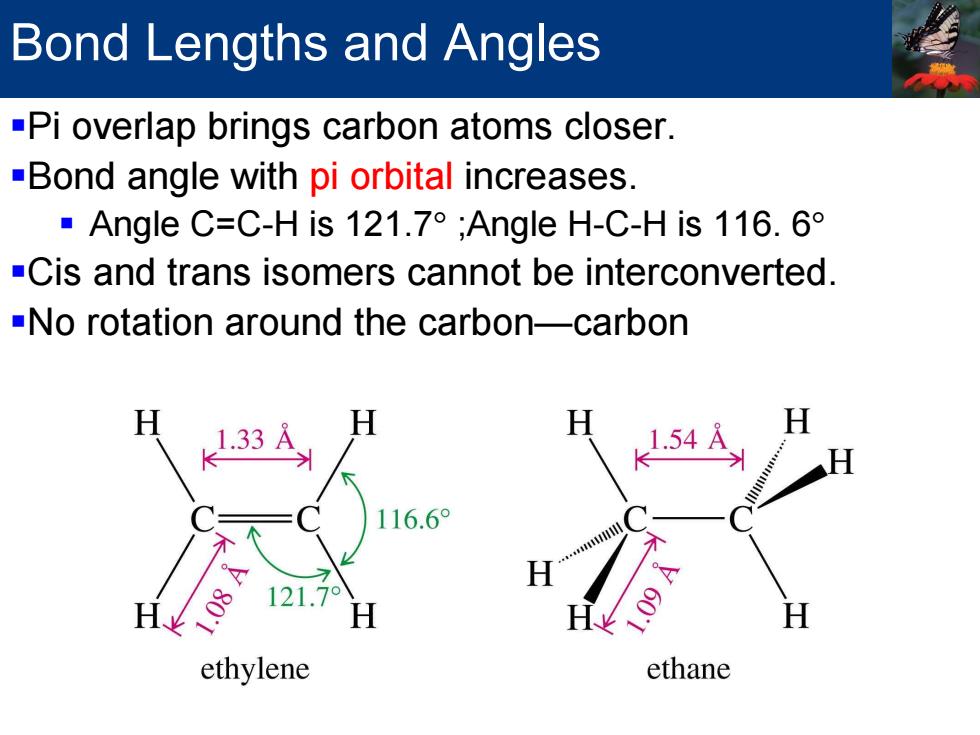
Bond Lengths and Angles -Pi overlap brings carbon atoms closer. -Bond angle with pi orbital increases. ·Angle C=C-His121.7°;Angle H-C-His116.6 -Cis and trans isomers cannot be interconverted -No rotation around the carbon-carbon 1.33 116.6° H ethylene ethane
Bond Lengths and Angles Pi overlap brings carbon atoms closer. Bond angle with pi orbital increases. Angle C=C-H is 121.7° ;Angle H-C-H is 116. 6° Cis and trans isomers cannot be interconverted. No rotation around the carbon—carbon
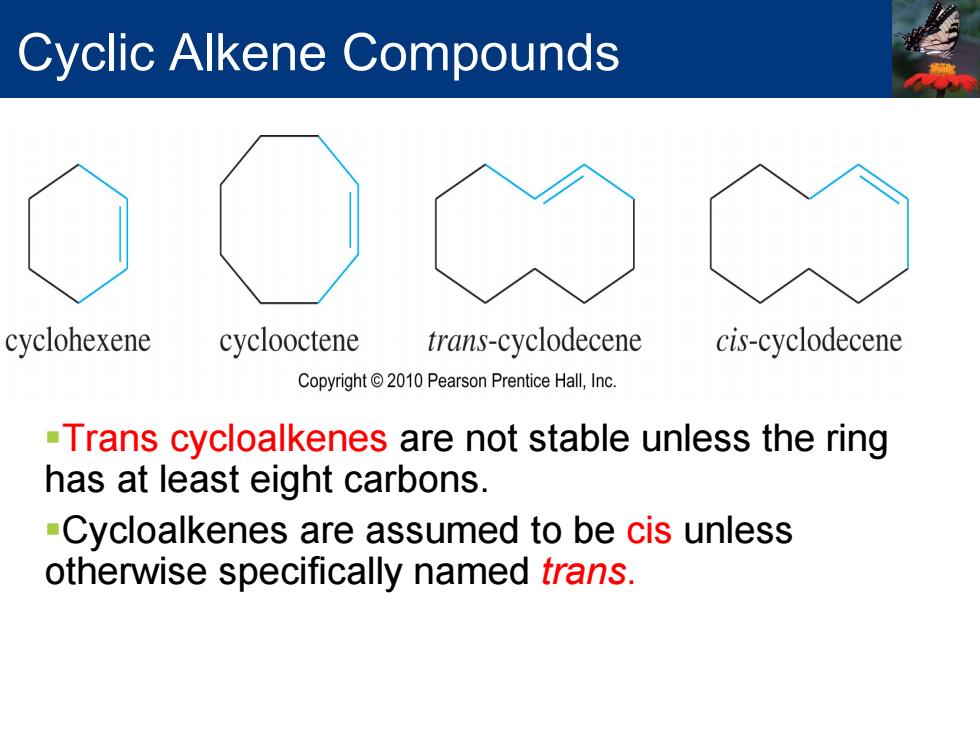
Cyclic Alkene Compounds cyclohexene cyclooctene trans-cyclodecene cis-cyclodecene Copyright 2010 Pearson Prentice Hall,Inc. -Trans cycloalkenes are not stable unless the ring has at least eight carbons. -Cycloalkenes are assumed to be cis unless otherwise specifically named frans
Cyclic Alkene Compounds Trans cycloalkenes are not stable unless the ring has at least eight carbons. Cycloalkenes are assumed to be cis unless otherwise specifically named trans

Stability of Alkenes Stability of cis and trans isomers Interconversion does not occur spontaneously but can be induced by strong acid H CH3 H3C、 CH3 Acid C=C catalyst H3C Trans (76%) Cis(24%) Steric strain cis-But-2-ene trans-But-2-ene
Stability of cis and trans isomers • Interconversion does not occur spontaneously but can be induced by strong acid Stability of Alkenes