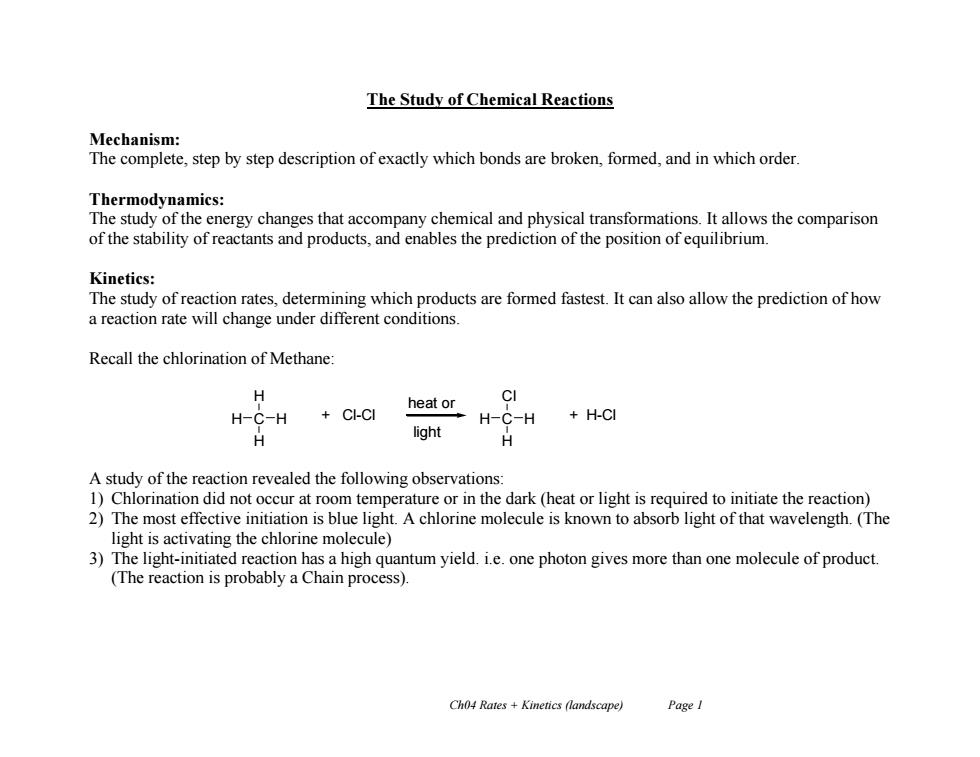
The Study of Chemical Reactions Mechanism: The complete,step by step description of exactly which bonds are broken,formed,and in which order. Thermodynamics: The study of the energy changes that accompany chemical and physical transformations.It allows the comparison of the stability of reactants and products,and enables the prediction of the position of equilibrium. Kinetics: The study of reaction rates,determining which products are formed fastest.It can also allow the prediction of how a reaction rate will change under different conditions. Recall the chlorination of Methane: CI heat or H-C-H CI-CI H-C-H +H-CI light H A study of the reaction revealed the following observations: 1)Chlorination did not occur at room temperature or in the dark (heat or light is required to initiate the reaction) 2)The most effective initiation is blue light.A chlorine molecule is known to absorb light of that wavelength.(The light is activating the chlorine molecule) 3)The light-initiated reaction has a high quantum yield.i.e.one photon gives more than one molecule of product. (The reaction is probably a Chain process). Ch04 Rates +Kinetics (landscape) PageI
Ch04 Rates + Kinetics (landscape) Page 1 The Study of Chemical Reactions Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order. Thermodynamics: The study of the energy changes that accompany chemical and physical transformations. It allows the comparison of the stability of reactants and products, and enables the prediction of the position of equilibrium. Kinetics: The study of reaction rates, determining which products are formed fastest. It can also allow the prediction of how a reaction rate will change under different conditions. Recall the chlorination of Methane: A study of the reaction revealed the following observations: 1) Chlorination did not occur at room temperature or in the dark (heat or light is required to initiate the reaction) 2) The most effective initiation is blue light. A chlorine molecule is known to absorb light of that wavelength. (The light is activating the chlorine molecule) 3) The light-initiated reaction has a high quantum yield. i.e. one photon gives more than one molecule of product. (The reaction is probably a Chain process). H H C H H + Cl-Cl Cl H C H H + H-Cl heat or light
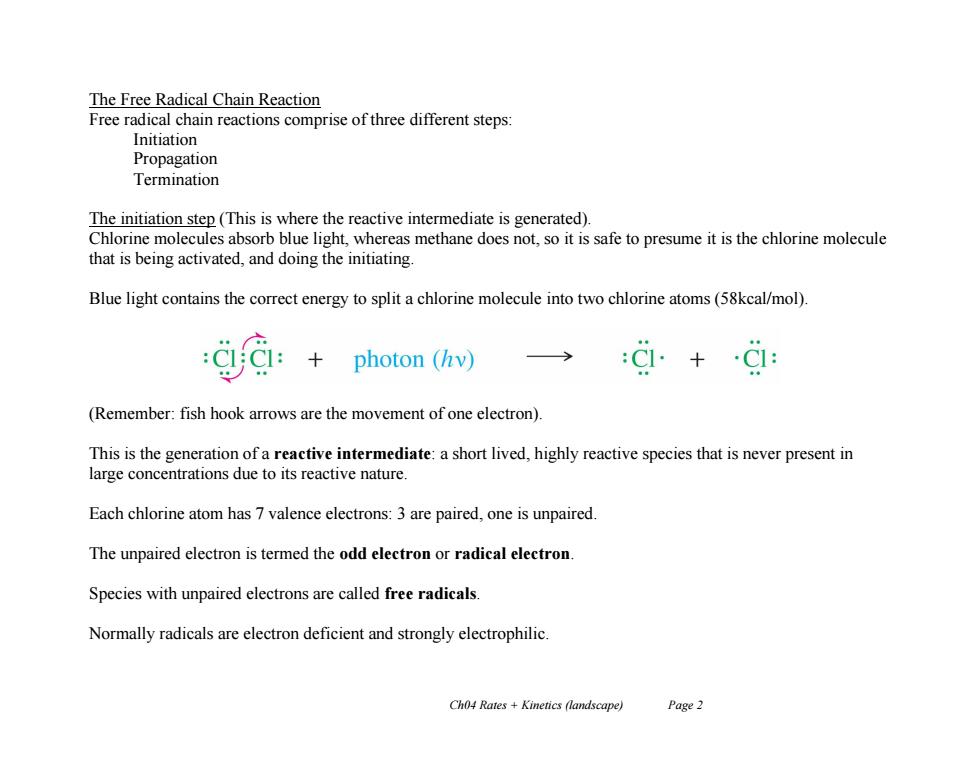
The Free Radical Chain Reaction Free radical chain reactions comprise of three different steps: Initiation Propagation Termination The initiation step (This is where the reactive intermediate is generated). Chlorine molecules absorb blue light,whereas methane does not,so it is safe to presume it is the chlorine molecule that is being activated,and doing the initiating. Blue light contains the correct energy to split a chlorine molecule into two chlorine atoms(58kcal/mol). photon (hv) → (Remember:fish hook arrows are the movement of one electron) This is the generation of a reactive intermediate:a short lived,highly reactive species that is never present in large concentrations due to its reactive nature. Each chlorine atom has 7 valence electrons:3 are paired,one is unpaired. The unpaired electron is termed the odd electron or radical electron Species with unpaired electrons are called free radicals. Normally radicals are electron deficient and strongly electrophilic. Ch04 Rates Kinetics (landscape) Page 2
Ch04 Rates + Kinetics (landscape) Page 2 The Free Radical Chain Reaction Free radical chain reactions comprise of three different steps: Initiation Propagation Termination The initiation step (This is where the reactive intermediate is generated). Chlorine molecules absorb blue light, whereas methane does not, so it is safe to presume it is the chlorine molecule that is being activated, and doing the initiating. Blue light contains the correct energy to split a chlorine molecule into two chlorine atoms (58kcal/mol). (Remember: fish hook arrows are the movement of one electron). This is the generation of a reactive intermediate: a short lived, highly reactive species that is never present in large concentrations due to its reactive nature. Each chlorine atom has 7 valence electrons: 3 are paired, one is unpaired. The unpaired electron is termed the odd electron or radical electron. Species with unpaired electrons are called free radicals. Normally radicals are electron deficient and strongly electrophilic
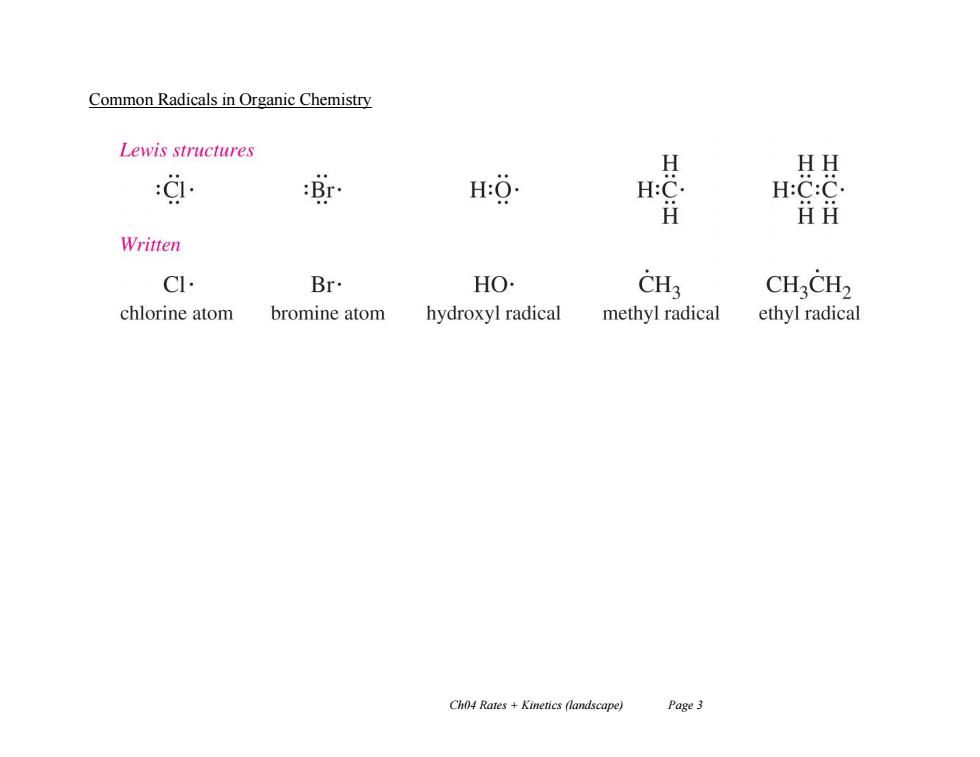
Common Radicals in Organic Chemistry Lewis structures H HH :过 r H:O. H:C. H:C:C. H 丑H Written C Br HO CH3 CHCH2 chlorine atom bromine atom hydroxyl radical methyl radical ethyl radical Ch04 Rates +Kinetics (landscape) Page 3
Ch04 Rates + Kinetics (landscape) Page 3 Common Radicals in Organic Chemistry
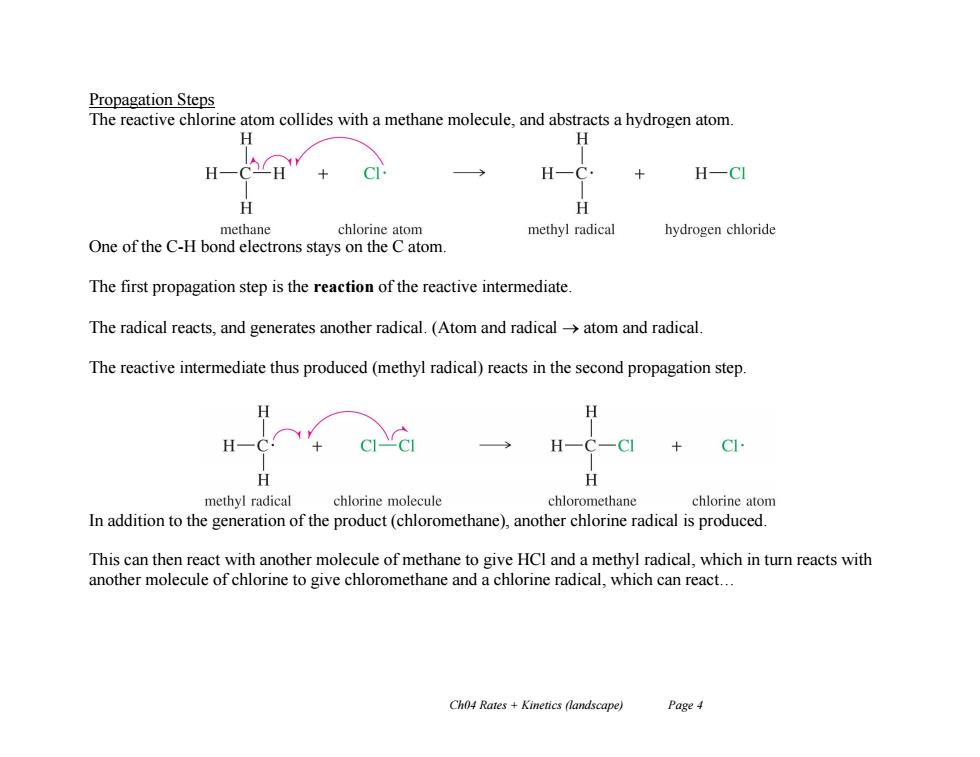
Propagation Steps The reactive chlorine atom collides with a methane molecule,and abstracts a hydrogen atom H H H-CH C H一 H-CI H H methane chlorine atom methyl radical hydrogen chloride One of the C-H bond electrons stays on the C atom. The first propagation step is the reaction of the reactive intermediate. The radical reacts,and generates another radical.(Atom and radical->atom and radical The reactive intermediate thus produced(methyl radical)reacts in the second propagation step. H C H methyl radical chlorine molecule chloromethane chlorine atom In addition to the generation of the product(chloromethane),another chlorine radical is produced. This can then react with another molecule of methane to give HCl and a methyl radical,which in turn reacts with another molecule of chlorine to give chloromethane and a chlorine radical,which can react... Ch04 Rates +Kinetics (landscape) Page 4
Ch04 Rates + Kinetics (landscape) Page 4 Propagation Steps The reactive chlorine atom collides with a methane molecule, and abstracts a hydrogen atom. One of the C-H bond electrons stays on the C atom. The first propagation step is the reaction of the reactive intermediate. The radical reacts, and generates another radical. (Atom and radical atom and radical. The reactive intermediate thus produced (methyl radical) reacts in the second propagation step. In addition to the generation of the product (chloromethane), another chlorine radical is produced. This can then react with another molecule of methane to give HCl and a methyl radical, which in turn reacts with another molecule of chlorine to give chloromethane and a chlorine radical, which can react…
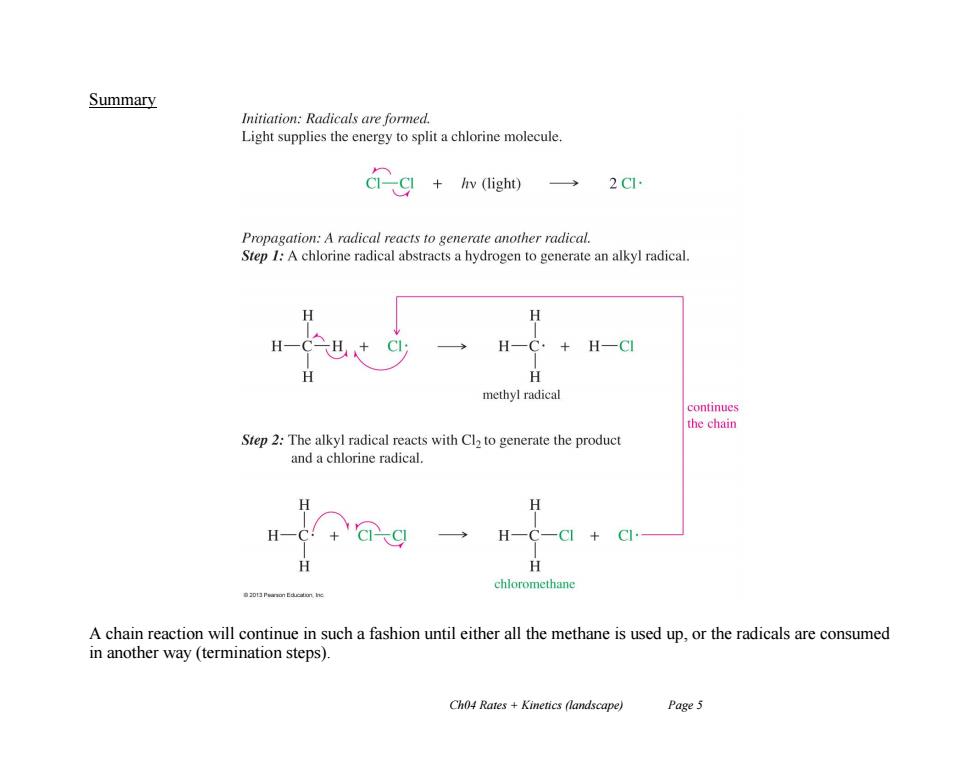
Summary Initiation:Radicals are formed. Light supplies the energy to split a chlorine molecule. Gg+wigh一 Propagation:A radical reacts to generate another radical. Step I:A chlorine radical abstracts a hydrogen to generate an alkyl radical. H-C. +HCI H methyl radical continues the chain Step 2:The alkyl radical reacts with Cl2 to generate the product and a chlorine radical. H H H chloromethane 20Pearmn Eaucatunt he A chain reaction will continue in such a fashion until either all the methane is used up,or the radicals are consumed in another way (termination steps). Ch04 Rates +Kinetics (landscape) Page 5
Ch04 Rates + Kinetics (landscape) Page 5 Summary A chain reaction will continue in such a fashion until either all the methane is used up, or the radicals are consumed in another way (termination steps)
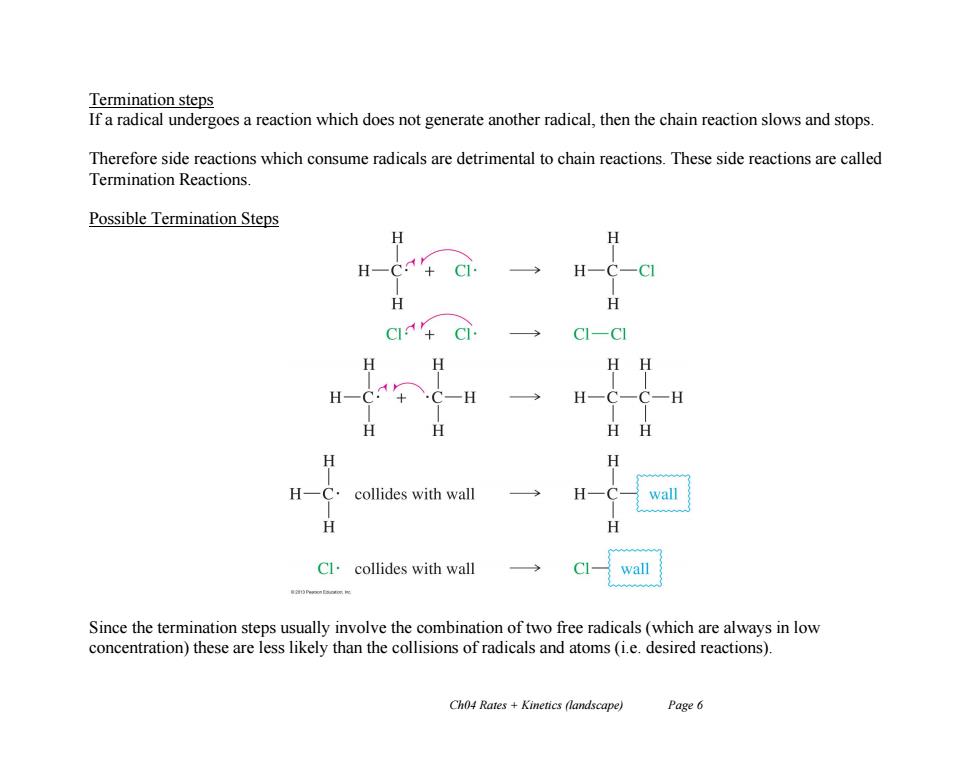
Termination steps If a radical undergoes a reaction which does not generate another radical,then the chain reaction slows and stops. Therefore side reactions which consume radicals are detrimental to chain reactions.These side reactions are called Termination Reactions. Possible Termination Steps H H-C H H -C] H HH H collides with wall wall Cl.collides with wall CI- wall Since the termination steps usually involve the combination of two free radicals(which are always in low concentration)these are less likely than the collisions of radicals and atoms (i.e.desired reactions). Ch04 Rates Kinetics (landscape) Page 6
Ch04 Rates + Kinetics (landscape) Page 6 Termination steps If a radical undergoes a reaction which does not generate another radical, then the chain reaction slows and stops. Therefore side reactions which consume radicals are detrimental to chain reactions. These side reactions are called Termination Reactions. Possible Termination Steps Since the termination steps usually involve the combination of two free radicals (which are always in low concentration) these are less likely than the collisions of radicals and atoms (i.e. desired reactions)
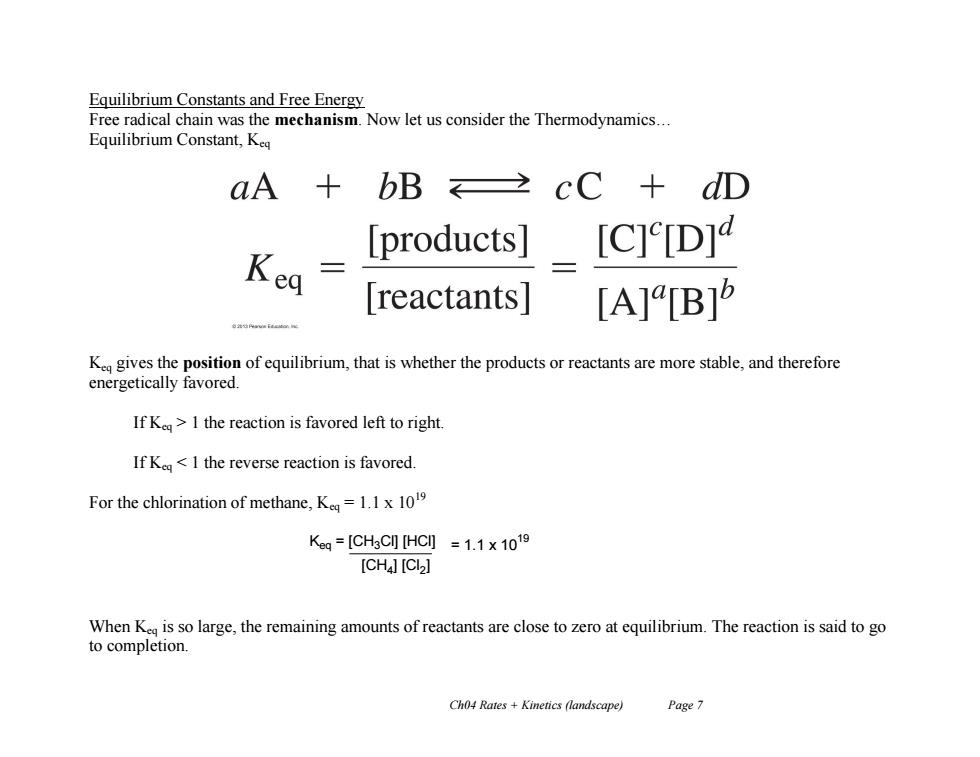
Equilibrium Constants and Free Energy Free radical chain was the mechanism.Now let us consider the Thermodynamics... Equilibrium Constant,Keq aA bB cC+dD products] [C]e[D] Kea reactants| [A][B]5 K gives the position of equilibrium,that is whether the products or reactants are more stable,and therefore energetically favored. If Ke>I the reaction is favored left to right. If Kea] When K is so large,the remaining amounts of reactants are close to zero at equilibrium.The reaction is said to go to completion. Ch04 Rates Kinetics (landscape) Page 7
Ch04 Rates + Kinetics (landscape) Page 7 Equilibrium Constants and Free Energy Free radical chain was the mechanism. Now let us consider the Thermodynamics… Equilibrium Constant, Keq Keq gives the position of equilibrium, that is whether the products or reactants are more stable, and therefore energetically favored. If Keq > 1 the reaction is favored left to right. If Keq < 1 the reverse reaction is favored. For the chlorination of methane, Keq = 1.1 x 1019 When Keq is so large, the remaining amounts of reactants are close to zero at equilibrium. The reaction is said to go to completion. Keq = [CH3Cl] [HCl] [CH4 ] [Cl2 ] = 1.1 x 10 19
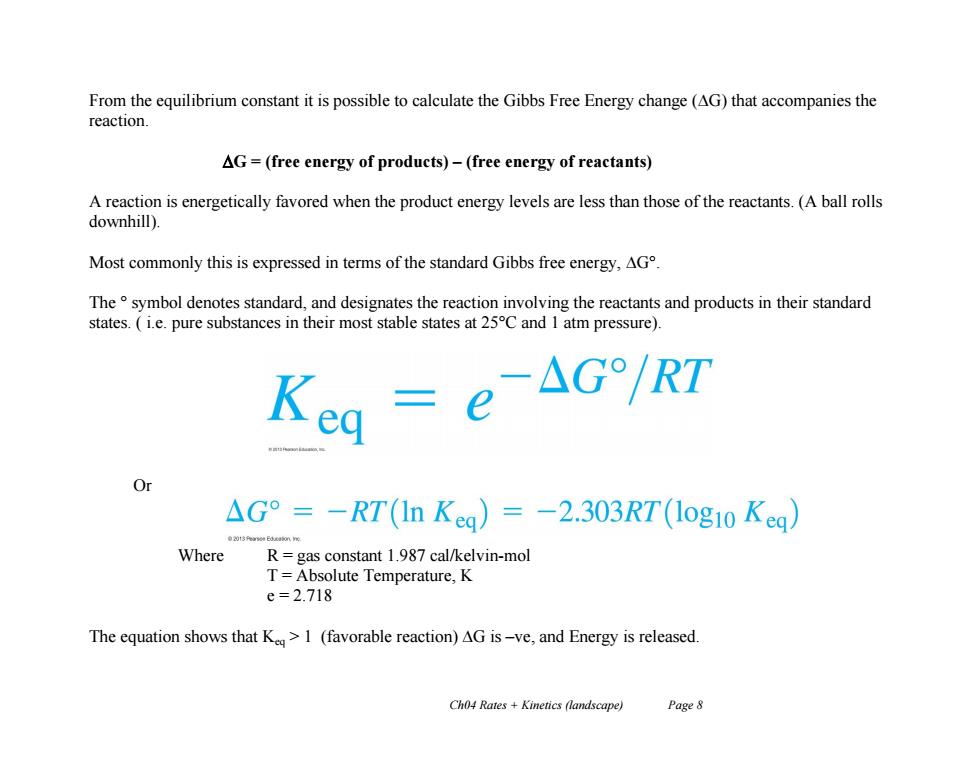
From the equilibrium constant it is possible to calculate the Gibbs Free Energy change (AG)that accompanies the reaction. AG=(free energy of products)-(free energy of reactants) A reaction is energetically favored when the product energy levels are less than those of the reactants.(A ball rolls downhill). Most commonly this is expressed in terms of the standard Gibbs free energy,AG The symbol denotes standard,and designates the reaction involving the reactants and products in their standard states.(ie.pure substances in their most stable states at 25C and 1 atm pressure). △G°=-RT(In Keg)=-2.303RT(1og10Kea) Where R=gas constant 1.987 cal/kelvin-mol T=Absolute Temperature,K e=2.718 The equation shows that K>1 (favorable reaction)AG is-ve,and Energy is released. Ch04 Rates Kinetics (landscape) Page 8
Ch04 Rates + Kinetics (landscape) Page 8 From the equilibrium constant it is possible to calculate the Gibbs Free Energy change (G) that accompanies the reaction. G = (free energy of products) – (free energy of reactants) A reaction is energetically favored when the product energy levels are less than those of the reactants. (A ball rolls downhill). Most commonly this is expressed in terms of the standard Gibbs free energy, G°. The ° symbol denotes standard, and designates the reaction involving the reactants and products in their standard states. ( i.e. pure substances in their most stable states at 25°C and 1 atm pressure). Or Where R = gas constant 1.987 cal/kelvin-mol T = Absolute Temperature, K e = 2.718 The equation shows that Keq > 1 (favorable reaction) G is –ve, and Energy is released

Enthalpy and Entropy There are two factors that combine to give the free energy change for a reaction:Enthalpy(H)and Entropy(S). △G°=△H°.T△S° AH=change in enthalpy=(enthalpy of prods)-(enthalpy of reactants) AS=change in entropy =(entropy of prods)-(entropy of reactants) (Typically the AH term>>TAS term) Enthalpy The change in enthalpy is the heat evolved or consumed in a reaction,normally in kcal/mol. Reactions tend to favor products with stronger bonds(i.e.lower enthalpy). AH is-ve,exothermic reaction,heat is evolved,stronger bonds are formed. AH is +ve,endothermic reaction,heat is consumed,weaker bonds are formed. A-ve AH is a favorable contribution to the free energy change. A +ve AH is an unfavorable contribution to the free energy change The AH for chlorination of methane is-25kcal/mol.(Very exothermic) Ch04 Rates Kinetics (landscape) Page 9
Ch04 Rates + Kinetics (landscape) Page 9 Enthalpy and Entropy There are two factors that combine to give the free energy change for a reaction: Enthalpy (H) and Entropy (S). G° = H° - TS° H° = change in enthalpy = (enthalpy of prods) – (enthalpy of reactants) S° = change in entropy = (entropy of prods) – (entropy of reactants) (Typically the H term >> TS term) Enthalpy The change in enthalpy is the heat evolved or consumed in a reaction, normally in kcal/mol. Reactions tend to favor products with stronger bonds (i.e. lower enthalpy). H is –ve, exothermic reaction, heat is evolved, stronger bonds are formed. H is +ve, endothermic reaction, heat is consumed, weaker bonds are formed. A –ve H is a favorable contribution to the free energy change. A +ve H is an unfavorable contribution to the free energy change. The H° for chlorination of methane is –25kcal/mol. (Very exothermic)

Entropy Entropy is often described as randomness,or freedom to move,or disorder. Reactions tend to favor products with greatest entropy Note the sign of the entropy contribution to AG: A +ve AS term makes a favorable contribution to the free energy. For the chlorination of methane,AS=2.9 entropy units(eu) So -T△S°=-298Kx2.9=-0.86kcal/mol Remember△H°=-25kcal,(much larger) So△G°=-25-0.86=-26kcal/mol (Often AG is approximated to be equal to AH). In kJ... △G°=△H°-T△S°=-105.1kJ/mol-3.61kJ/mol =-108.7 kJ/mol (-25.9 kcal/mol) Ch04 Rates +Kinetics (landscape) Page 10
Ch04 Rates + Kinetics (landscape) Page 10 Entropy Entropy is often described as randomness, or freedom to move, or disorder. Reactions tend to favor products with greatest entropy. Note the sign of the entropy contribution to G: A +ve S term makes a favorable contribution to the free energy. For the chlorination of methane, S° = 2.9 entropy units (eu) So -TS° = -298K x 2.9 = -0.86kcal/mol Remember H° = -25kcal, (much larger) So G° = -25 - 0.86 =-26kcal/mol. (Often G is approximated to be equal to H). In kJ…