
CNGNGE JOHN MCMURRY CHAPTER12 Organohalides: Nucleophilic Substitutions and Eliminations Organic Chemistry with Biological applications
CHAPTER12 Organohalides: Nucleophilic Substitutions and Eliminations
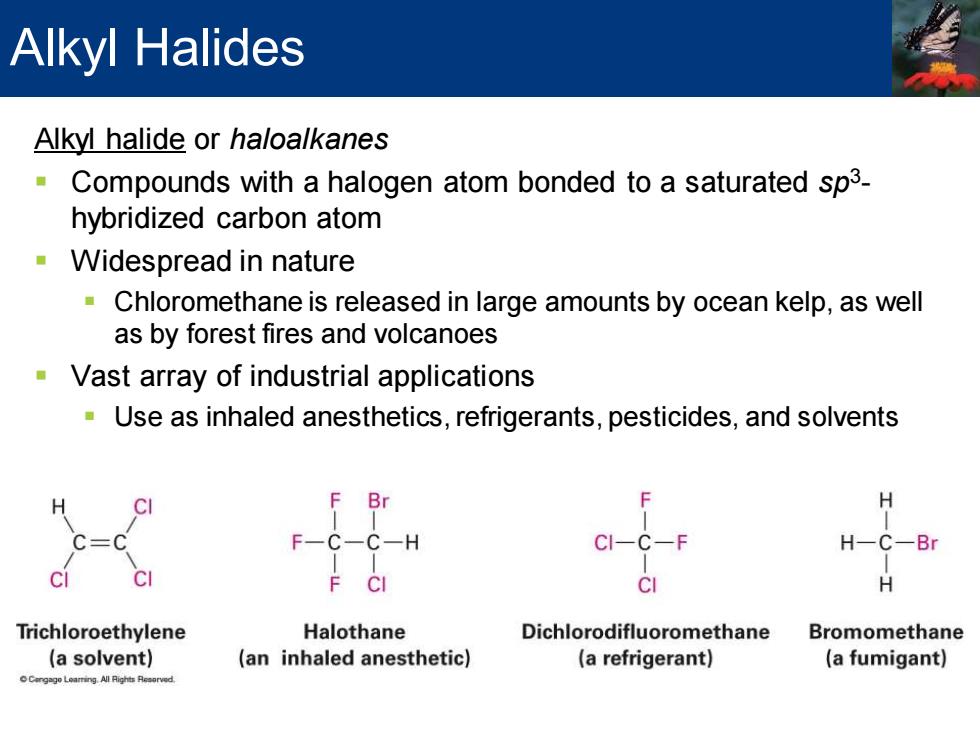
Alkyl Halides Alkyl halide or haloalkanes Compounds with a halogen atom bonded to a saturated sp3- hybridized carbon atom Widespread in nature Chloromethane is released in large amounts by ocean kelp,as well as by forest fires and volcanoes Vast array of industrial applications Use as inhaled anesthetics,refrigerants,pesticides,and solvents F Br H C三 F-C-C-H CI-C-F H-C-Br CI F CI CI H Trichloroethylene Halothane Dichlorodifluoromethane Bromomethane (a solvent) (an inhaled anesthetic) (a refrigerant) (a fumigant) Leang All Rights Reaorved
Alkyl halide or haloalkanes ▪ Compounds with a halogen atom bonded to a saturated sp3 - hybridized carbon atom ▪ Widespread in nature ▪ Chloromethane is released in large amounts by ocean kelp, as well as by forest fires and volcanoes ▪ Vast array of industrial applications ▪ Use as inhaled anesthetics, refrigerants, pesticides, and solvents Alkyl Halides
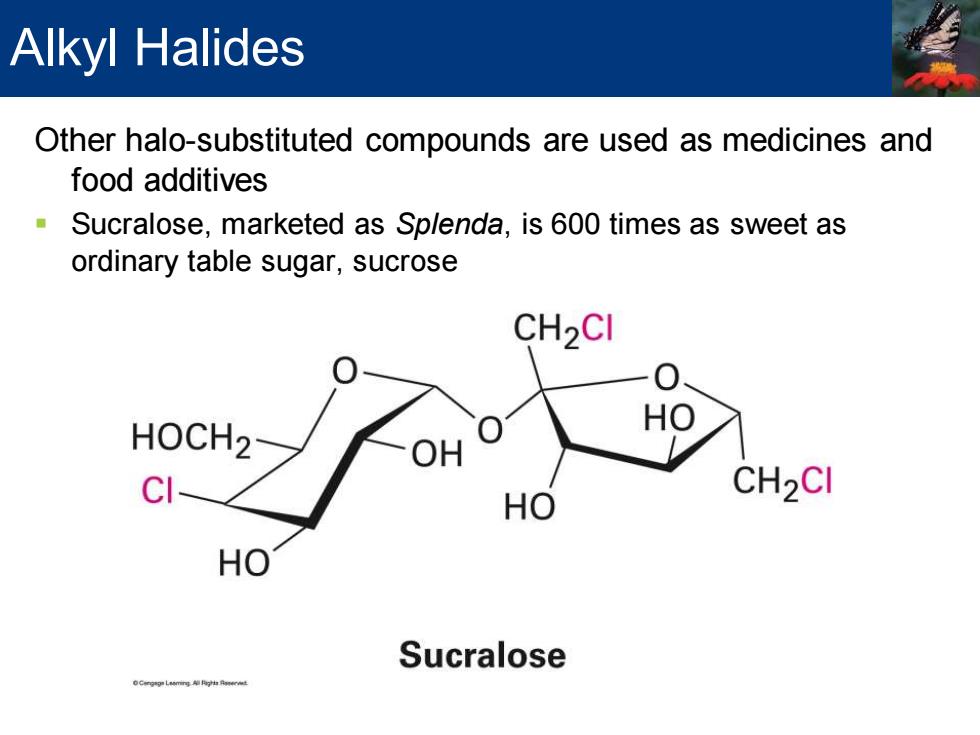
Alkyl Halides Other halo-substituted compounds are used as medicines and food additives Sucralose,marketed as Splenda,is 600 times as sweet as ordinary table sugar,sucrose CH2CI HOCH2 CI CH2CI HO HO Sucralose
Other halo-substituted compounds are used as medicines and food additives ▪ Sucralose, marketed as Splenda, is 600 times as sweet as ordinary table sugar, sucrose Alkyl Halides
Alkyl Halides Alkyl halides are not often involved in the biochemical pathways of terrestrial organisms The kinds of reactions they undergo-nucleophilic substitutions and eliminations -are frequently involved Alkyl halide chemistry acts as a relatively simple model for many mechanistically similar but structurally more complex reactions found in biomolecules
Alkyl halides are not often involved in the biochemical pathways of terrestrial organisms ▪ The kinds of reactions they undergo – nucleophilic substitutions and eliminations – are frequently involved ▪ Alkyl halide chemistry acts as a relatively simple model for many mechanistically similar but structurally more complex reactions found in biomolecules Alkyl Halides

12-1 Naming Alkyl Halides Haloalkanes 0 Commonly called alkyl halides 。 The halogen is treated as a substituent on a parent alkane chain Alkyl halides can be named by following three steps: Step 1 Find the longest chain,and name it as the parent 。 If a double or triple bond is present,it must be included in the parent chain
Haloalkanes • Commonly called alkyl halides • The halogen is treated as a substituent on a parent alkane chain Alkyl halides can be named by following three steps: Step 1 Find the longest chain, and name it as the parent • If a double or triple bond is present, it must be included in the parent chain 12-1 Naming Alkyl Halides
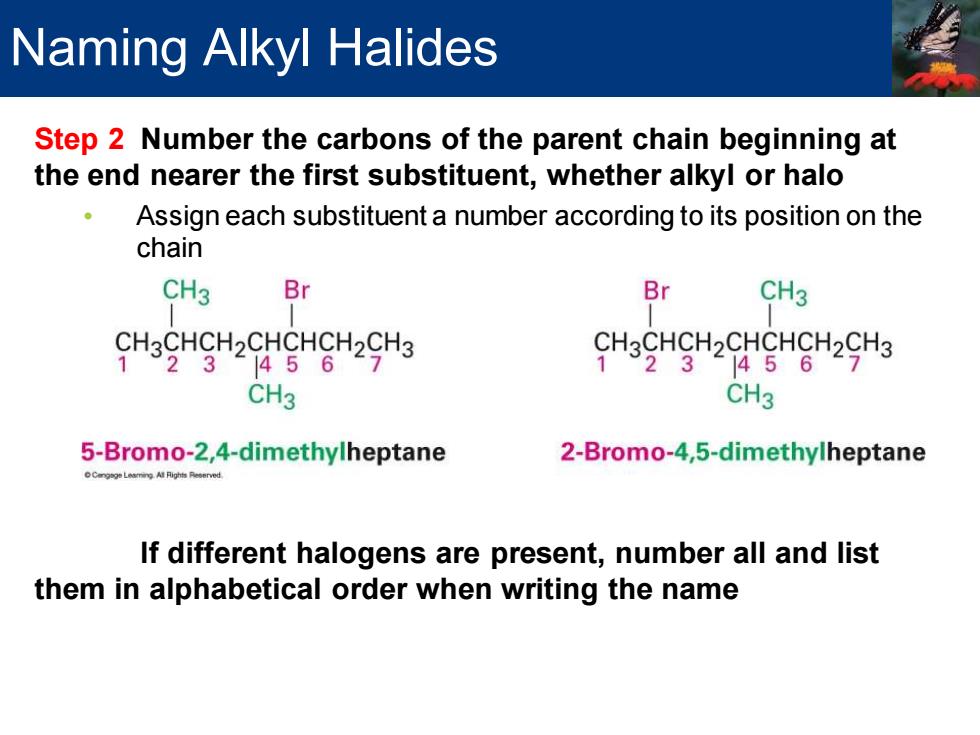
Naming Alkyl Halides Step 2 Number the carbons of the parent chain beginning at the end nearer the first substituent,whether alkyl or halo Assign each substituent a number according to its position on the chain CH3 Br Br CH3 CH3CHCH2CHCHCH2CH3 CH3CHCH2CHCHCH2CH3 1°231456 7 1231 1456 CH3 CH3 5-Bromo-2,4-dimethylheptane 2-Bromo-4,5-dimethylheptane If different halogens are present,number all and list them in alphabetical order when writing the name
Step 2 Number the carbons of the parent chain beginning at the end nearer the first substituent, whether alkyl or halo • Assign each substituent a number according to its position on the chain If different halogens are present, number all and list them in alphabetical order when writing the name Naming Alkyl Halides
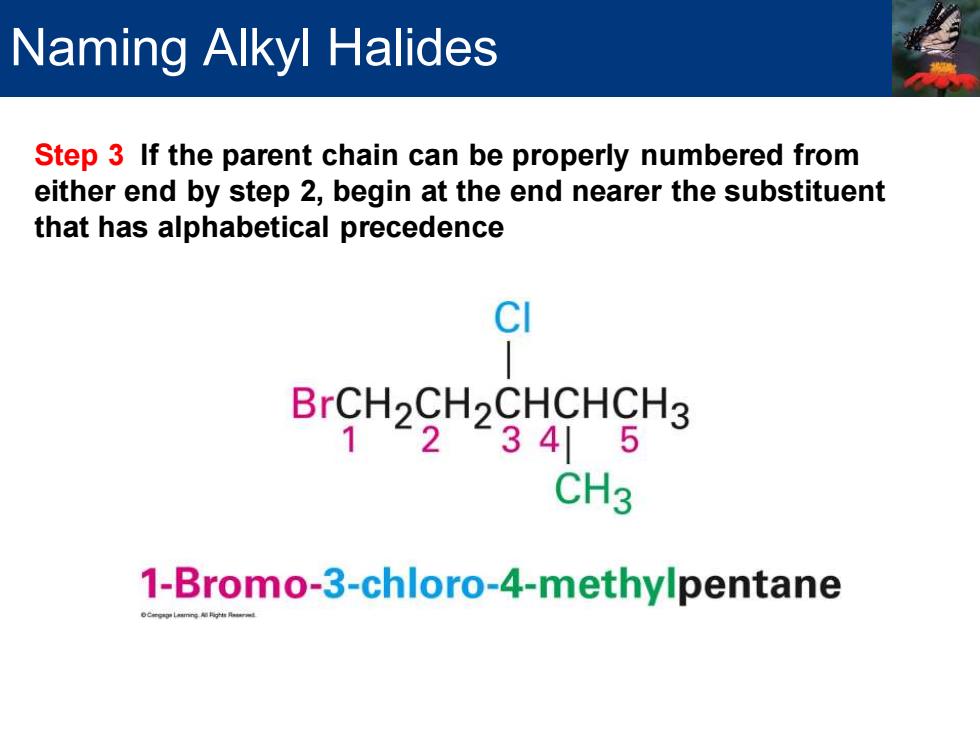
Naming Alkyl Halides Step 3 If the parent chain can be properly numbered from either end by step 2,begin at the end nearer the substituent that has alphabetical precedence CI BrCH2CH2CHCHCH3 1234|5 CH3 1-Bromo-3-chloro-4-methylpentane
Step 3 If the parent chain can be properly numbered from either end by step 2, begin at the end nearer the substituent that has alphabetical precedence Naming Alkyl Halides
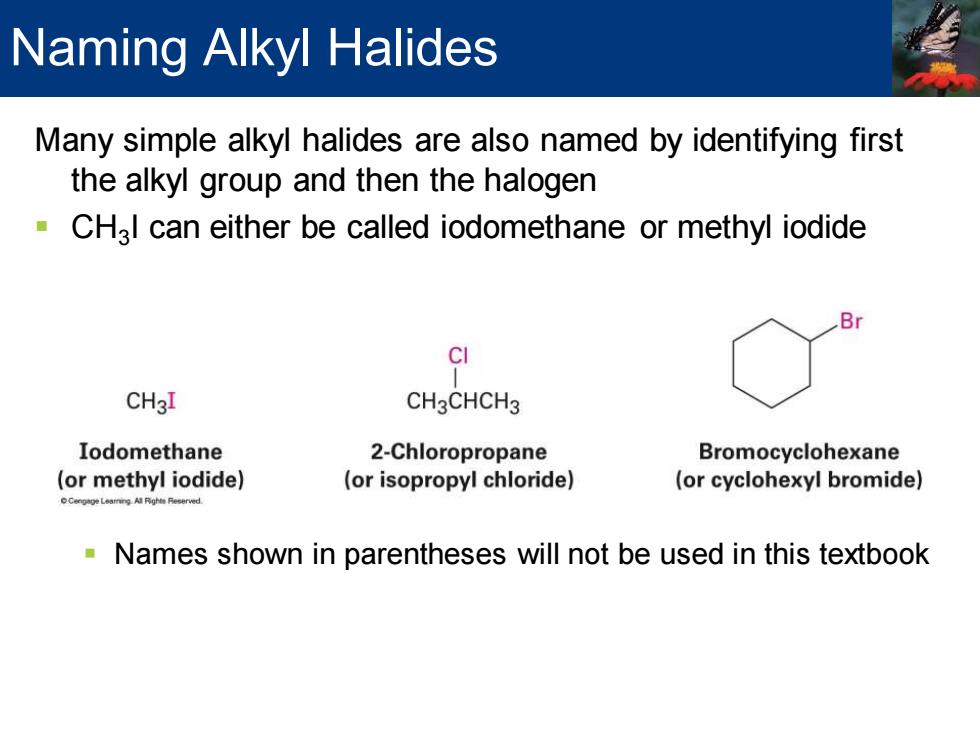
Naming Alkyl Halides Many simple alkyl halides are also named by identifying first the alkyl group and then the halogen CH3l can either be called iodomethane or methyl iodide B CI CH3I CH3CHCH3 Iodomethane 2-Chloropropane Bromocyclohexane (or methyl iodide) (or isopropyl chloride) (or cyclohexyl bromide) Names shown in parentheses will not be used in this textbook
Many simple alkyl halides are also named by identifying first the alkyl group and then the halogen ▪ CH3 I can either be called iodomethane or methyl iodide ▪ Names shown in parentheses will not be used in this textbook Naming Alkyl Halides
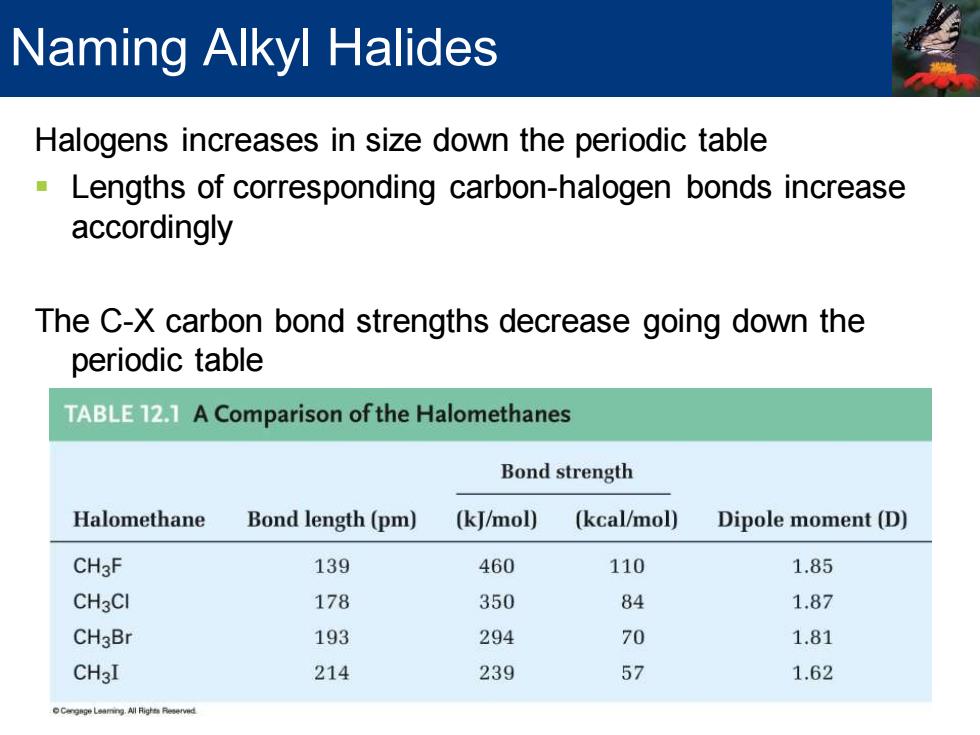
Naming Alkyl Halides Halogens increases in size down the periodic table Lengths of corresponding carbon-halogen bonds increase accordingly The C-X carbon bond strengths decrease going down the periodic table TABLE 12.1 A Comparison of the Halomethanes Bond strength Halomethane Bond length(pm) (kJ/mol) (kcal/mol) Dipole moment(D) CH3F 139 460 110 1.85 CH3CI 178 350 84 1.87 CH3Br 193 294 70 1.81 CH3I 214 239 57 1.62
Halogens increases in size down the periodic table ▪ Lengths of corresponding carbon-halogen bonds increase accordingly The C-X carbon bond strengths decrease going down the periodic table Naming Alkyl Halides
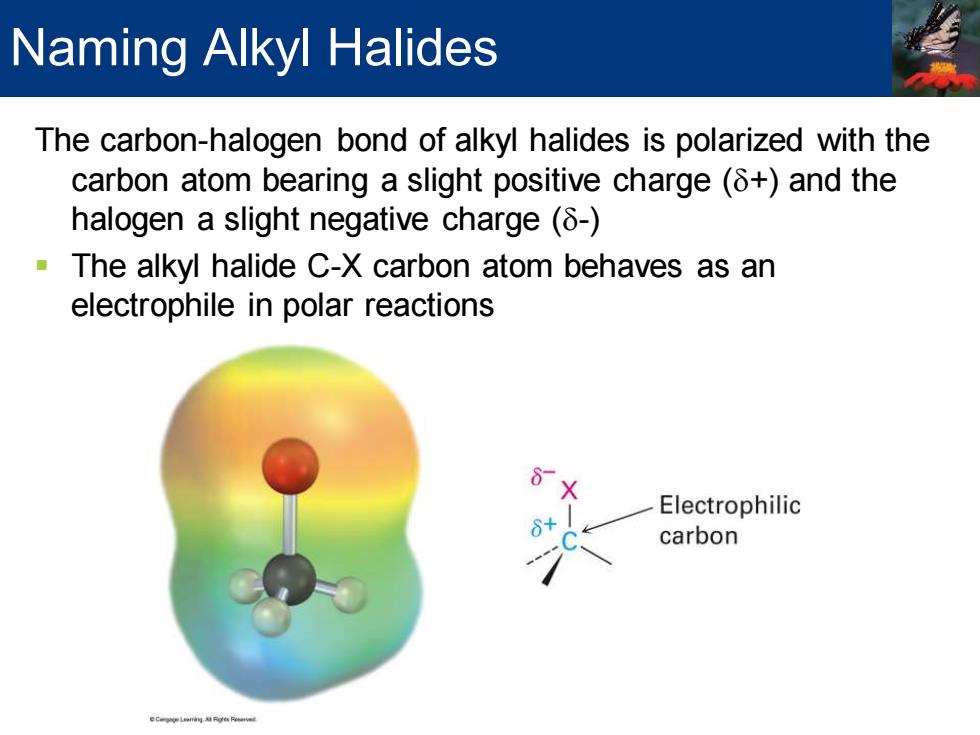
Naming Alkyl Halides The carbon-halogen bond of alkyl halides is polarized with the carbon atom bearing a slight positive charge(8+)and the halogen a slight negative charge(8-) The alkyl halide C-X carbon atom behaves as an electrophile in polar reactions Electrophilic carbon
The carbon-halogen bond of alkyl halides is polarized with the carbon atom bearing a slight positive charge (d+) and the halogen a slight negative charge (d-) ▪ The alkyl halide C-X carbon atom behaves as an electrophile in polar reactions Naming Alkyl Halides