
Organic Chemistry, ORGANIC 8th Edition CHEMISTRY L.G.Wade,Jr. Chapter 16 Lecture Aromatic Compounds 2013 Pearson Education,Inc
© 2013 Pearson Education, Inc. Chapter 16 1 Chapter 16 Lecture Organic Chemistry, 8 th Edition L. G. Wade, Jr. Aromatic Compounds © 2013 Pearson Education, Inc
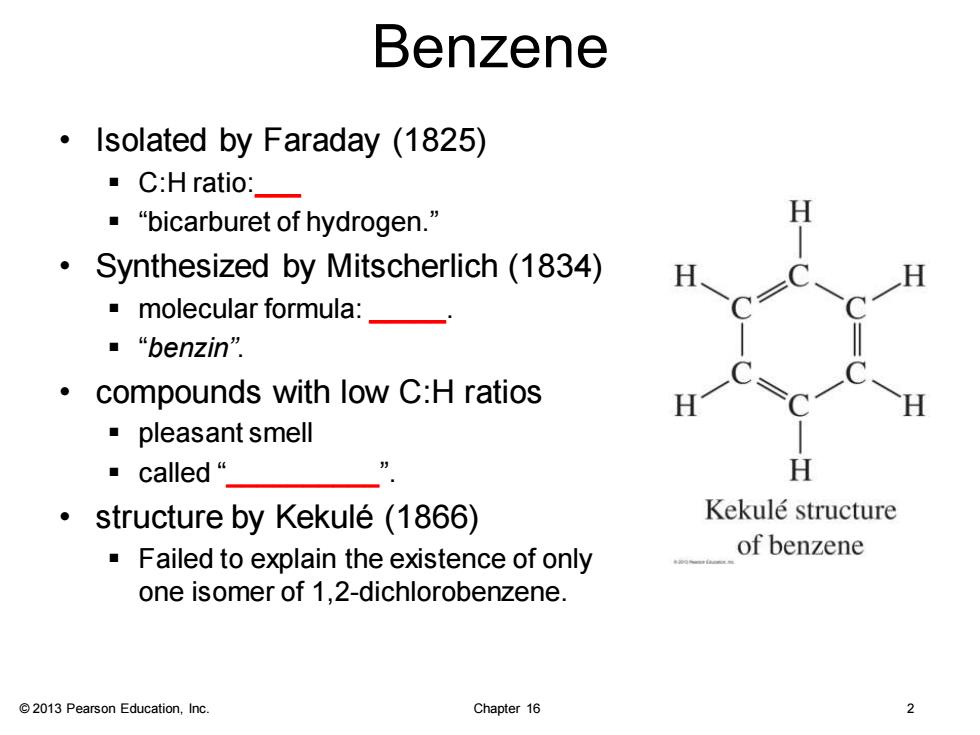
Benzene Isolated by Faraday (1825) ·C:H ratio:- ·“bicarburet of hydrogen.” Synthesized by Mitscherlich(1834) 。molecular formula:_— ■“benzin” compounds with low C:H ratios ·pleasant smell ·called“_ H structure by Kekule (1866) Kekule structure Failed to explain the existence of only of benzene one isomer of 1,2-dichlorobenzene. 2013 Pearson Education,Inc. Chapter 16 2
© 2013 Pearson Education, Inc. Chapter 16 2 Benzene • Isolated by Faraday (1825) ▪ C:H ratio:___ ▪ “bicarburet of hydrogen.” • Synthesized by Mitscherlich (1834) ▪ molecular formula: _____. ▪ “benzin”. • compounds with low C:H ratios ▪ pleasant smell ▪ called “__________”. • structure by Kekulé (1866) ▪ Failed to explain the existence of only one isomer of 1,2-dichlorobenzene
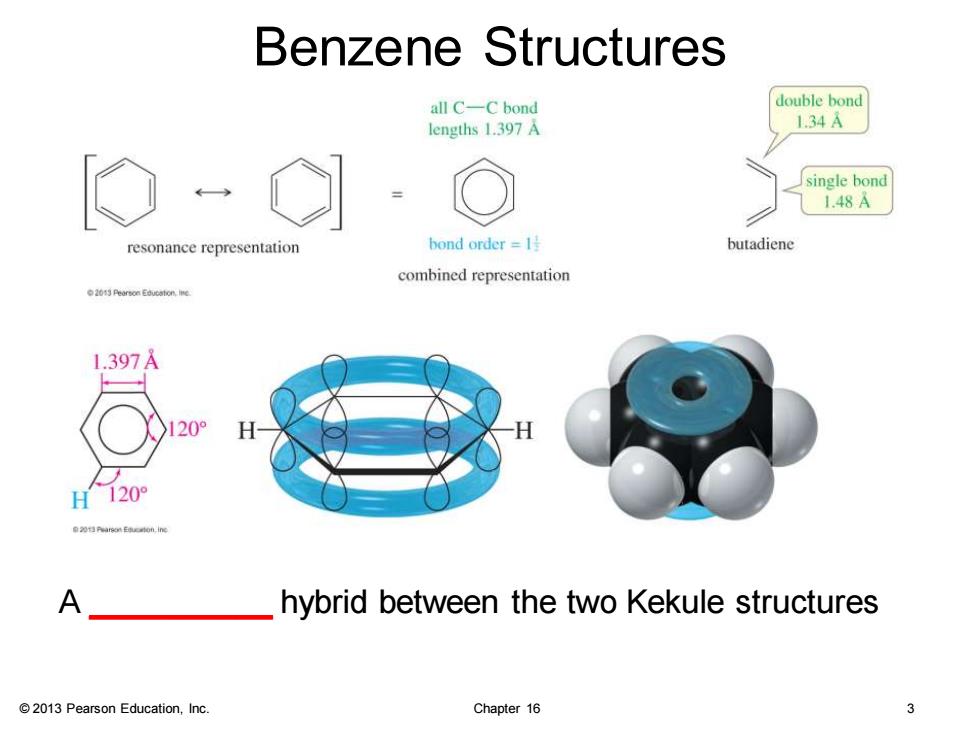
Benzene Structures all C-C bond double bond lengths 1.397A 134A single bond 1.48A resonance representation bond order =1 butadiene combined representation o20特earsonEdo 1.397A H120° A hybrid between the two Kekule structures 2013 Pearson Education,Inc. Chapter 16 3
© 2013 Pearson Education, Inc. Chapter 16 3 Benzene Structures A __________ hybrid between the two Kekule structures
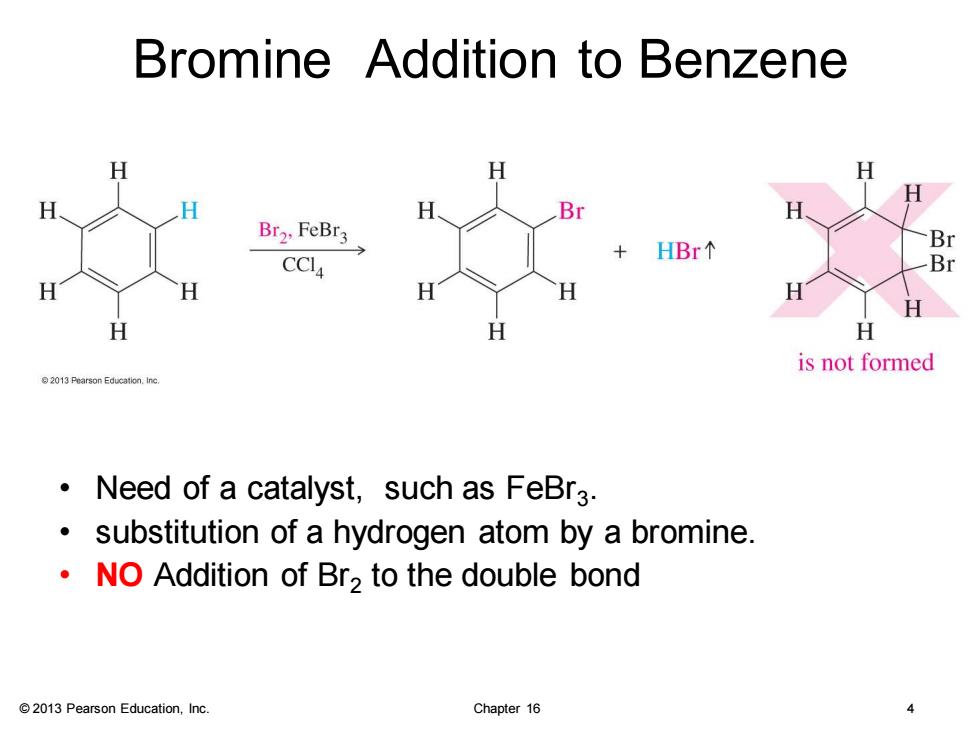
Bromine Addition to Benzene H H H H Br Br2,FeBr3 Br HBr个 CCI4 Br H H H H H H H is not formed 2013 Pearson Education.Inc. Need of a catalyst,such as FeBr3. substitution of a hydrogen atom by a bromine. NO Addition of Br2 to the double bond 2013 Pearson Education,Inc. Chapter 16 4
© 2013 Pearson Education, Inc. Chapter 16 4 Bromine Addition to Benzene • Need of a catalyst, such as FeBr3 . • substitution of a hydrogen atom by a bromine. • NO Addition of Br2 to the double bond
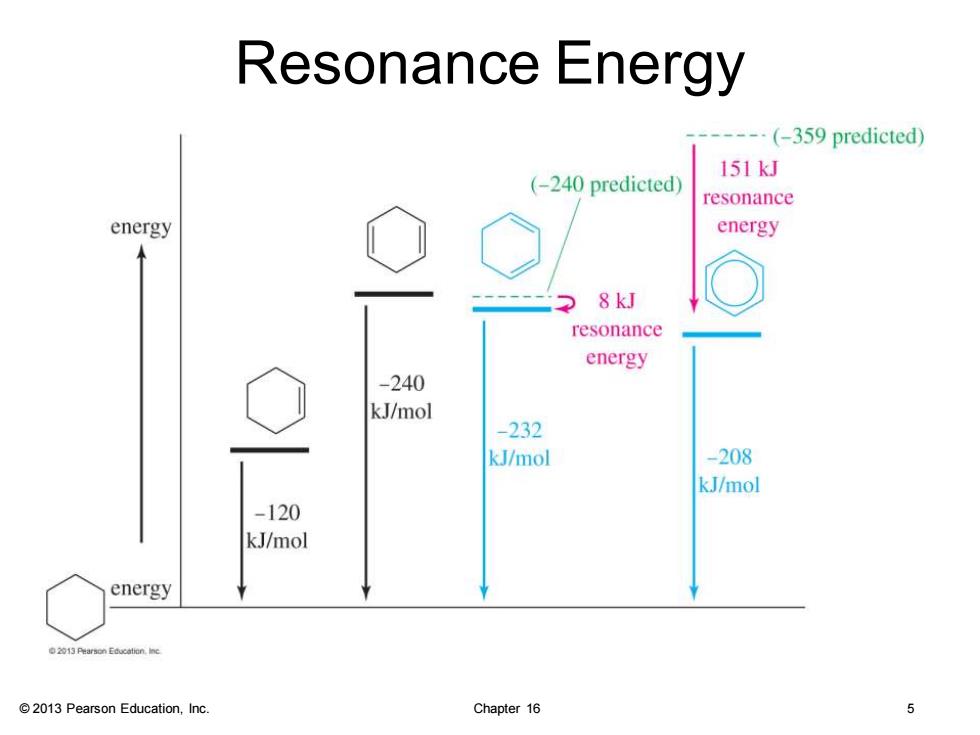
Resonance Energy ---(-359 predicted) 151kJ (-240 predicted) resonance energy energy 8kJ resonance energy -240 kJ/mol -232 kJ/mol -208 kJ/mol -120 kJ/mol energy 2013 Pearson Education,Inc. Chapter 16 5
© 2013 Pearson Education, Inc. Chapter 16 5 Resonance Energy
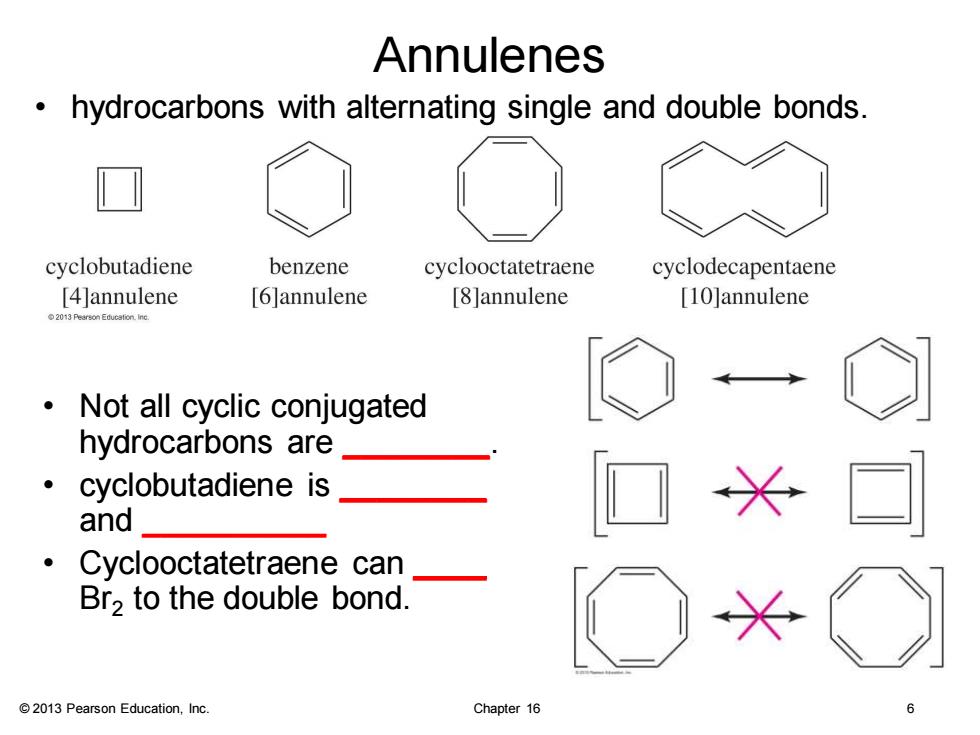
Annulenes hydrocarbons with alternating single and double bonds cyclobutadiene benzene cyclooctatetraene cyclodecapentaene [4]annulene [6]annulene [8]annulene [10]annulene 013 Pearson Education.Ine. Not all cyclic conjugated hydrocarbons are ● cyclobutadiene is and ● Cyclooctatetraene can Br2 to the double bond. 2013 Pearson Education,Inc. Chapter 16
© 2013 Pearson Education, Inc. Chapter 16 6 Annulenes • hydrocarbons with alternating single and double bonds. • Not all cyclic conjugated hydrocarbons are ________. • cyclobutadiene is ________ and __________ • Cyclooctatetraene can ____ Br2 to the double bond
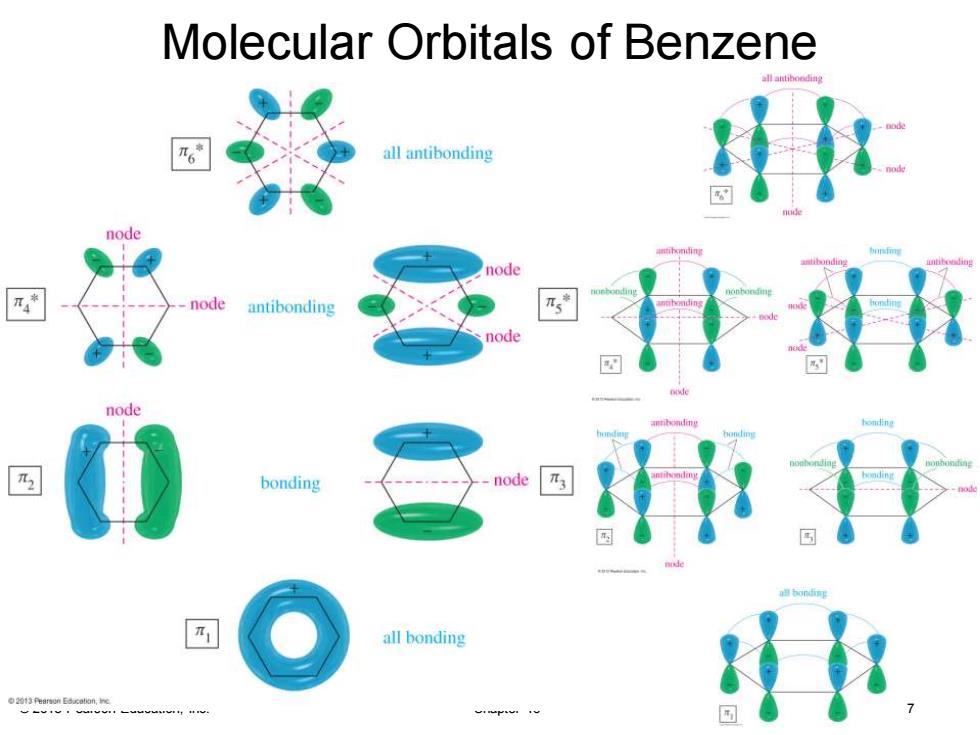
Molecular Orbitals of Benzene all antiboeding all antibonding node node node antibonding node node bonding node all bonding
© 2013 Pearson Education, Inc. Chapter 16 7 Molecular Orbitals of Benzene
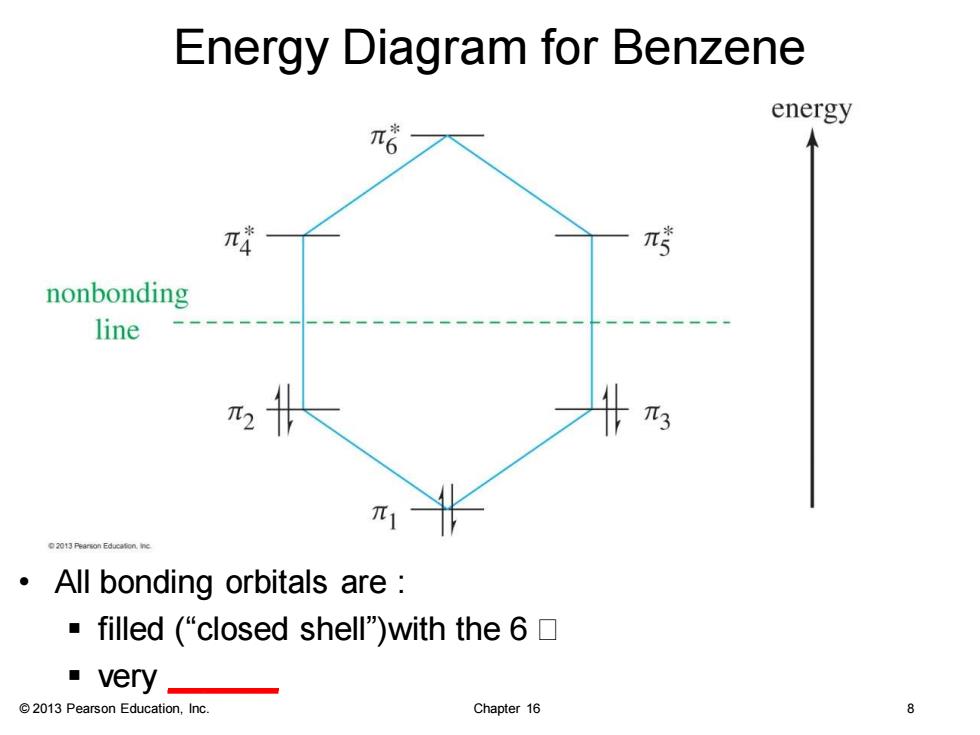
Energy Diagram for Benzene energy π哈 π4 nonbonding line π1 02013代arson Educaton nc All bonding orbitals are ·filled(“closed shell"with the6☐ very 2013 Pearson Education,Inc. Chapter 16 8
© 2013 Pearson Education, Inc. Chapter 16 8 Energy Diagram for Benzene • All bonding orbitals are : ▪ filled (“closed shell”)with the 6 ▪ very ______
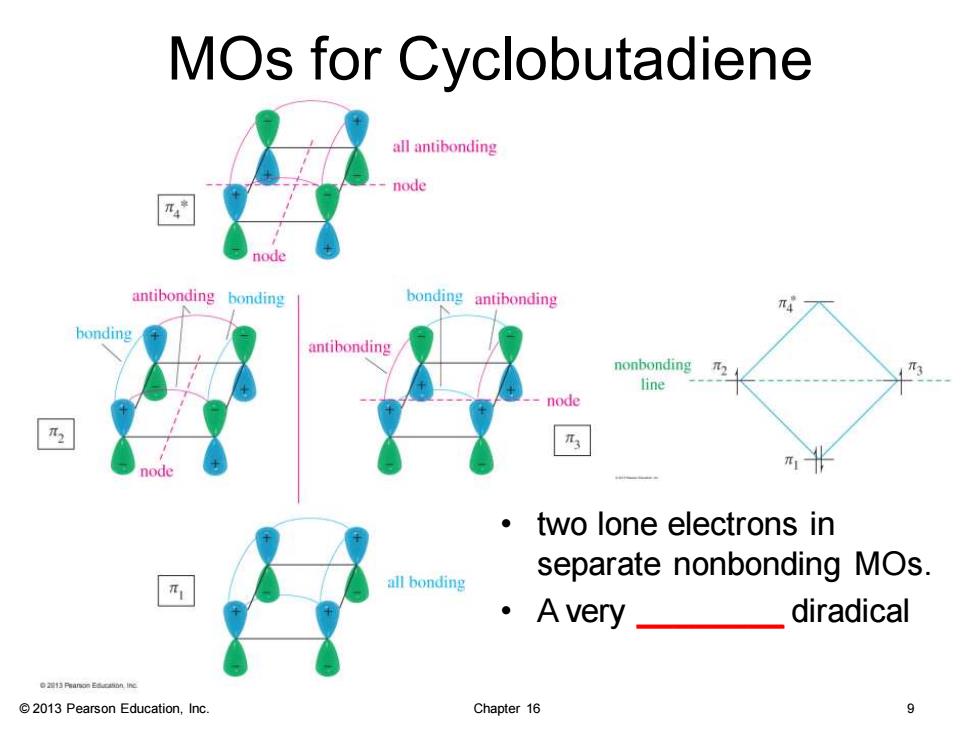
MOs for Cyclobutadiene all antibonding node node antibonding bonding bonding antibonding antibonding nonbonding line node ·two lone electrons in all bonding separate nonbonding MOs. ·Avey_ diradical e2接Prinon Eacaon Inc 2013 Pearson Education,Inc. Chapter 16 9
© 2013 Pearson Education, Inc. Chapter 16 9 MOs for Cyclobutadiene • two lone electrons in separate nonbonding MOs. • A very ________ diradical
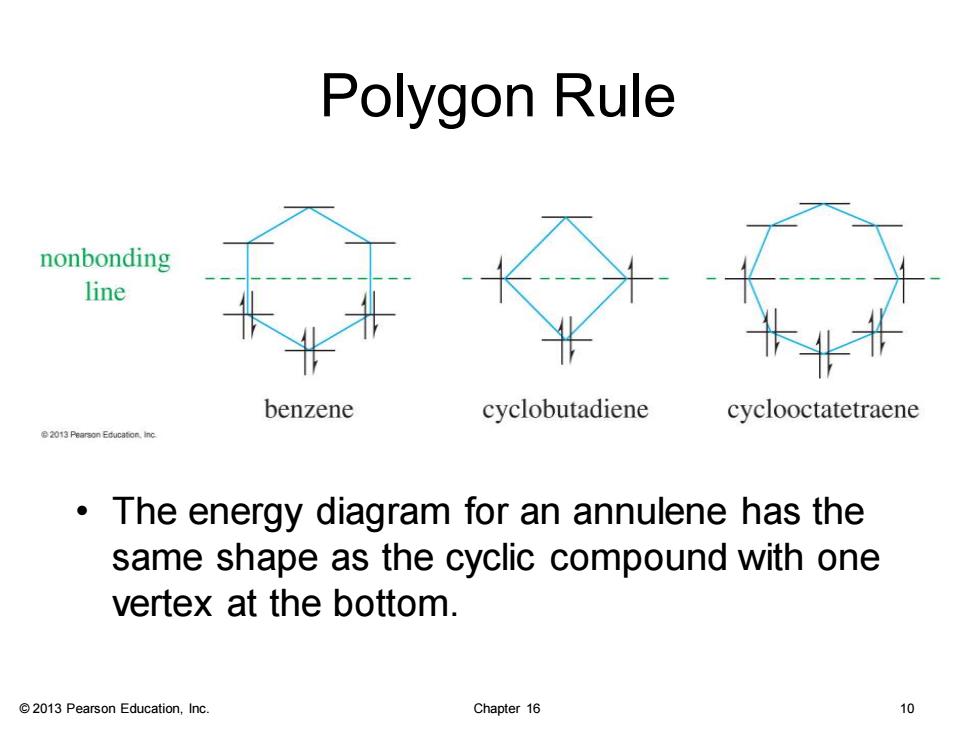
Polygon Rule nonbonding line benzene cyclobutadiene cyclooctatetraene 2013 Pearson Educaton.inc The energy diagram for an annulene has the same shape as the cyclic compound with one vertex at the bottom. 2013 Pearson Education,Inc. Chapter 16 0
© 2013 Pearson Education, Inc. Chapter 16 10 Polygon Rule • The energy diagram for an annulene has the same shape as the cyclic compound with one vertex at the bottom