
Organic Chemistry,7th Edition L.G.Wade,Jr. Chapter 24 Amino Acids,Peptides,and Proteins Copyright 2010 Pearson Education,Inc
Chapter 24 Copyright © 2010 Pearson Education, Inc. Organic Chemistry, 7th Edition L. G. Wade, Jr. Amino Acids, Peptides, and Proteins
Proteins Biopolymers of a-amino acids. -Amino acids are joined by peptide bond. They serve a variety of functions: ■Structure Enzymes Transport Protection ■Hormones Chapter 24 2
Chapter 24 2 Proteins ▪ Biopolymers of -amino acids. ▪ Amino acids are joined by peptide bond. ▪ They serve a variety of functions: ▪ Structure ▪ Enzymes ▪ Transport ▪ Protection ▪ Hormones
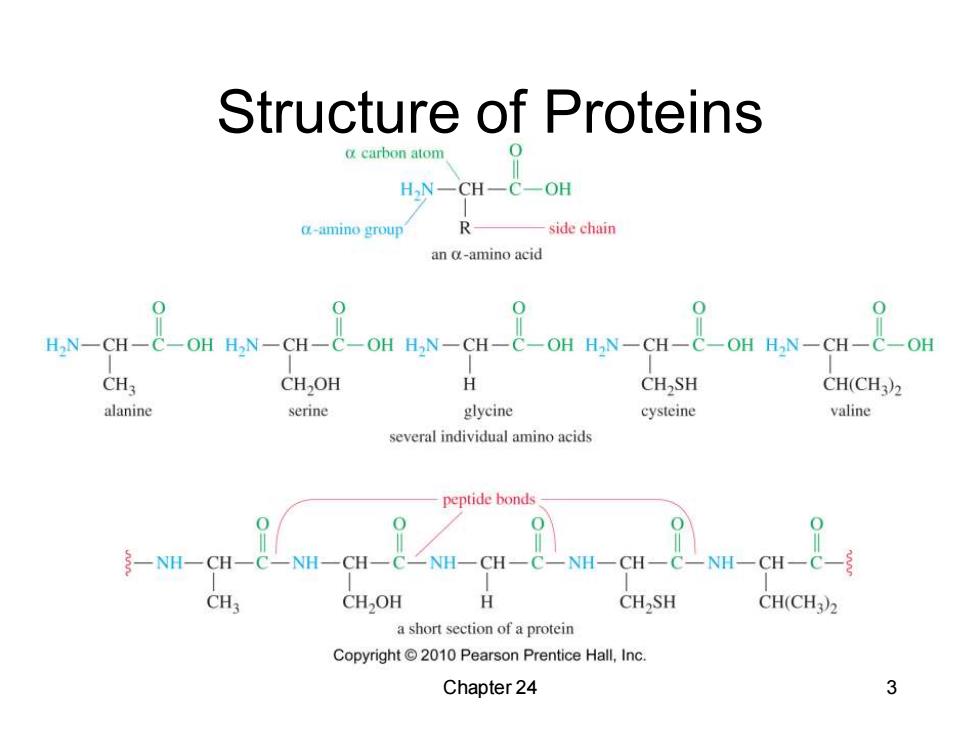
Structure of Proteins 仪carbon atom CH-C- OH a-amino group R —side chain an d-amino acid 0 0 HN-CH-C-OH H2N-CH-C-OH H2N-CH-C- OH HN-CH-C-OH HN-CH-C-OH CH3 CH2OH H CH2SH CH(CH3)2 alanine serine glycine cysteine valine several individual amino acids peptide bonds ;-NH-CH-C-NH-CH- NH一CH NH-CH- NH-CH-C -8 CH3 CH2OH H CH>SH CH(CH32 a short section of a protein Copyright 2010 Pearson Prentice Hall,Inc. Chapter 24 3
Chapter 24 3 Structure of Proteins
Amino Acids -NH2 on the carbon next to -COOH. Glycine,NH2-CH2-COOH,is simplest. -With-R side chain,molecule is chiral. -Most natural amino acids are L-amino acids,related to L-(-)-glyceraldehyde. Direction of optical rotation,(+)or(-), must be determined experimentally. Chapter 24
Chapter 24 4 Amino Acids ▪ —NH2 on the carbon next to —COOH. ▪ Glycine, NH2—CH2—COOH, is simplest. ▪ With —R side chain, molecule is chiral. ▪ Most natural amino acids are L-amino acids, related to L-(-)-glyceraldehyde. ▪ Direction of optical rotation, (+) or (-), must be determined experimentally
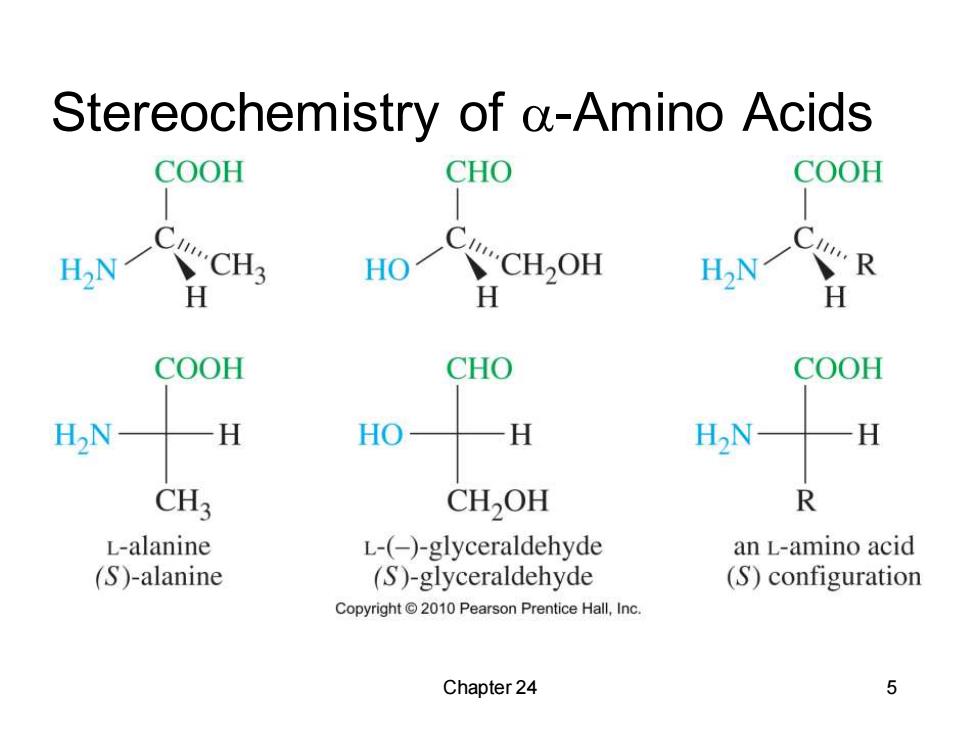
Stereochemistry of a-Amino Acids COOH CHO COOH H H H COOH CHO COOH H2N H HO H H2N- H CH3 CH2OH R L-alanine L-(-)-glyceraldehyde an L-amino acid (S)-alanine (S)-glyceraldehyde (S)configuration Copyright2010 Pearson Prentice Hall,Inc. Chapter 24 5
Chapter 24 5 Stereochemistry of -Amino Acids

Standard Amino Acids Twenty standard a-amino acids. Differ in side-chain characteristics: ·-H or alkyl ·Contains an-OH ■Contains sulfur Contains a nonbasic nitrogen Has -COOH -Has a basic nitrogen Chapter 24 6
Chapter 24 6 Standard Amino Acids ▪ Twenty standard -amino acids. ▪ Differ in side-chain characteristics: ▪ —H or alkyl ▪ Contains an —OH ▪ Contains sulfur ▪ Contains a nonbasic nitrogen ▪ Has —COOH ▪ Has a basic nitrogen
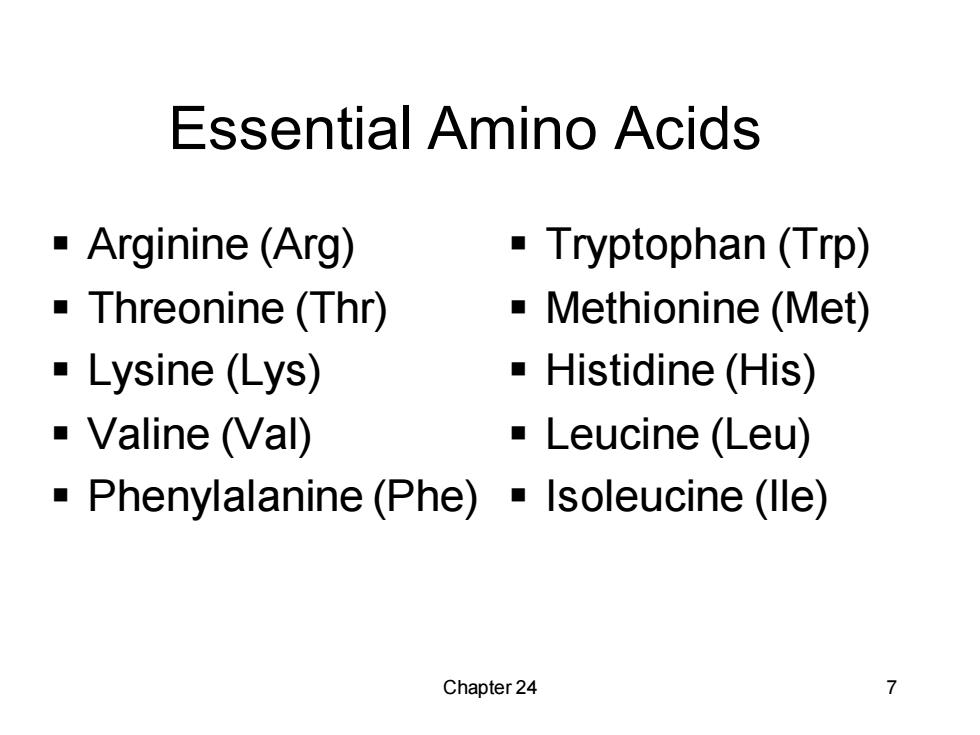
Essential Amino Acids Arginine (Arg) Tryptophan (Trp) -Threonine(Thr) Methionine (Met) Lysine (Lys) ■Histidine(His) Valine (Val) ·Leucine(Leu) -Phenylalanine(Phe)Isoleucine(lle) Chapter 24 7
Chapter 24 7 Essential Amino Acids ▪ Arginine (Arg) ▪ Threonine (Thr) ▪ Lysine (Lys) ▪ Valine (Val) ▪ Phenylalanine (Phe) ▪ Tryptophan (Trp) ▪ Methionine (Met) ▪ Histidine (His) ▪ Leucine (Leu) ▪ Isoleucine (Ile)
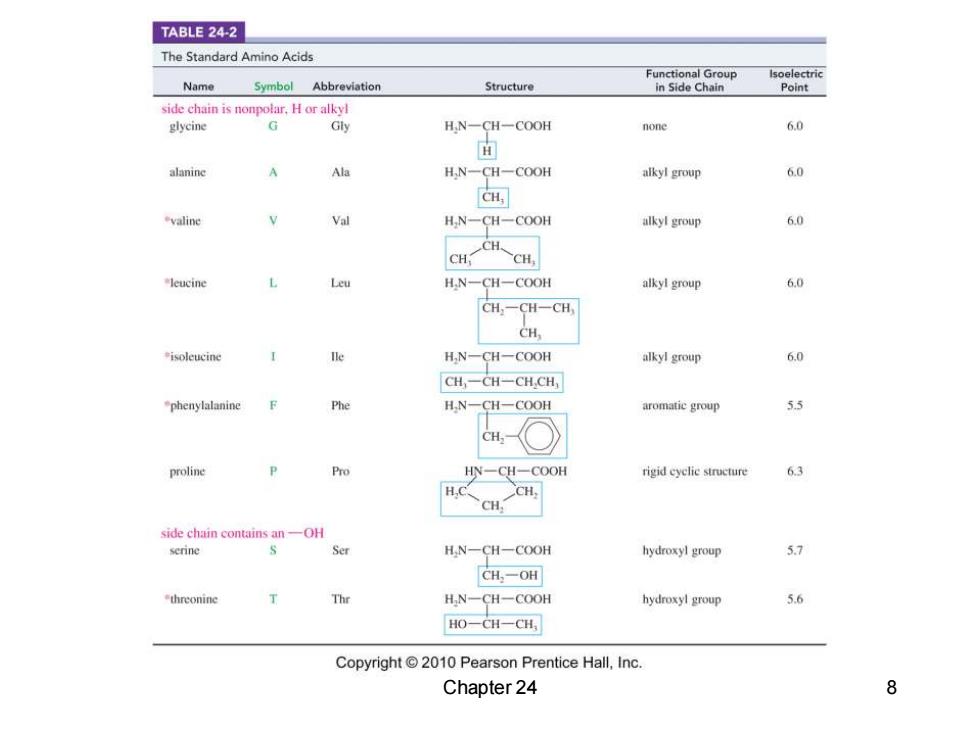
TABLE 24-2 The Standard Amino Acids Functiona Name Symbol Abbreviation Structure Point side chain is nonpolar,H or alkyl glycine G Gly HN-CH一COOH none 6.0 面 alaninc Ala H.N-CH-COOH alkyl group 6.0 H valine Val HN-CH-COOH alkyl group 6.0 leucine Leu H,N一CH一COOH alkyl group 6.0 CH,一CH-CH, CH, isoleucine H,N-CH-COOH alkyl group 6.0 CH,-CH一CH.CH1 phenylalanine Phe H,N-CH-COOH aromatic group 5.5 CH,- proline Pro HN-CH一COOH rigid cyclie structure 6.3 H. CH, CH. side chain contains an-OH serine Ser H,N-CH一COOH hydroxyl group 57 CH-OH threonine Thr H,N-CH-COOH hydroxyl group 5.6 HO-CH一CH Copyright 2010 Pearson Prentice Hall,Inc. Chapter 24 8
Chapter 24 8
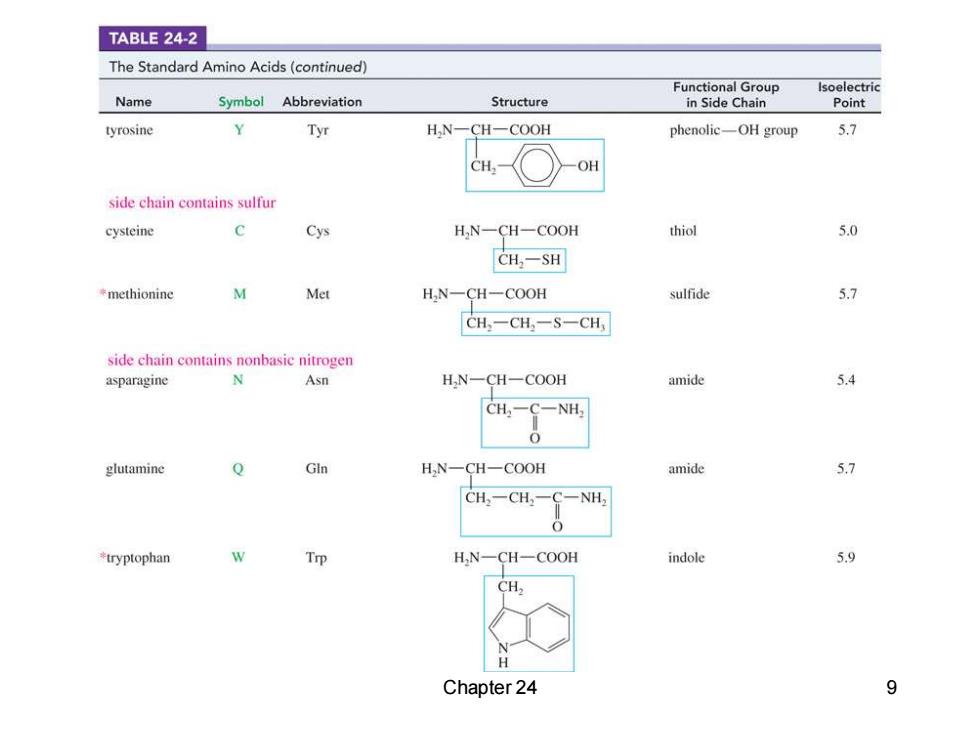
TABLE 24-2 The Standard Amino Acids(continued) Functional Group lsoelectric Name Symbol Abbreviation Structure in Side Chain Point tyrosine Tyr H,N一CH-COOH phenolic-OH group 5.7 CH, OH side chain contains sulfur cysteine c Cys H,N-CH-COOH thiol 5.0 CH,-SH methionine W Met H,N-CH-COOH sulfide 5.7 CH一CH一S-CH side chain contains nonbasic nitrogen asparagine N Asn HN-CH一COOH amide 5.4 CH2-C-NH2 0 glutamine GIn H,N-CH-COOH amide 5.7 CH,一CH C一NH tryptophan W Trp H,N-CH-COOH indole 5.9 H H Chapter 24 9
Chapter 24 9
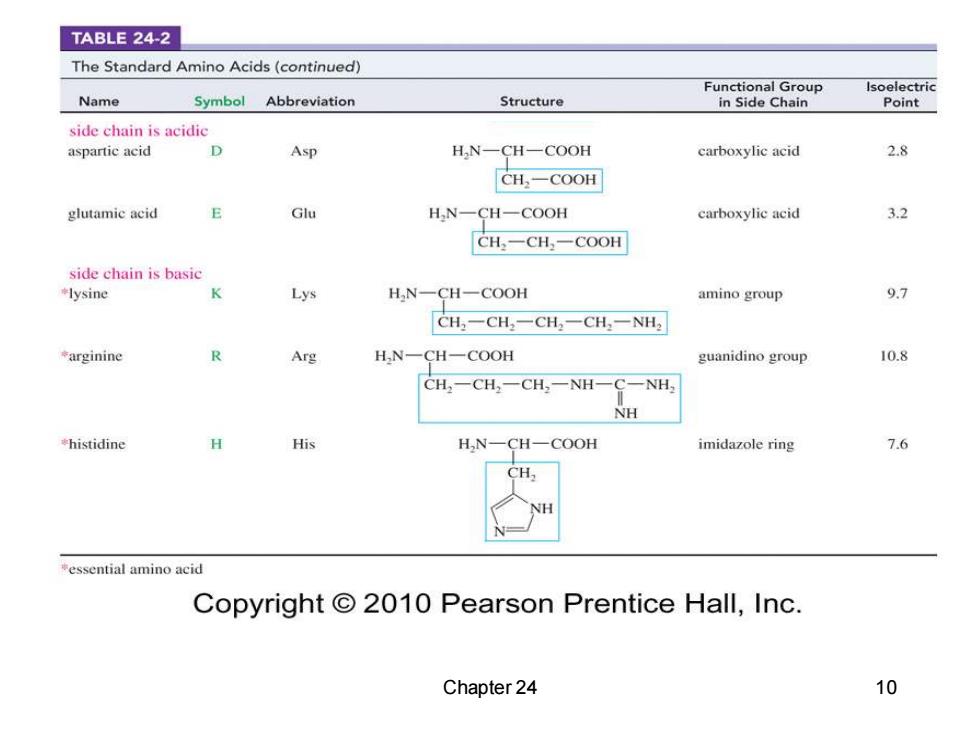
TABLE 24-2 The Standard Amino Acids(continued) Functional Group Isoelectric Name Symbol Abbreviation Structure in Side Chain Point side chain is acidic aspartic acid Asp H,N-CH-COOH carboxylic acid 2.8 CH,-COOH glutamic acid E Glu H,N- CH一COOH carboxylic acid 3.2 CH一CH一COOH side chain is basic lysine Lys H,N-CH-COOH amino group CH,-CH,-CH,-CH,一NH, arginine Arg H,N一CH一COOH guanidino group 10.8 CH2一CH2一CH,-NH C-NH, NH histidine His H,N-CH-COOH imidazole ring 7.6 essential amino acid Copyright 2010 Pearson Prentice Hall,Inc. Chapter 24 10
Chapter 24 10