
Organic Chemistry,7th Edition H. L.G.Wade,Jr. H Chapter 4 The Study of Chemical Reactions Copyright 2010 Pearson Education,Inc
Chapter 4 Copyright © 2010 Pearson Education, Inc. Organic Chemistry, 7th Edition L. G. Wade, Jr. The Study of Chemical Reactions

Introduction ·Overall reaction:reactants→products Mechanism:Step-by-step pathway. To learn more about a reaction: ·Thermodynamics Kinetics Chapter 4 2
Chapter 4 2 Introduction • Overall reaction: reactants → products • Mechanism: Step-by-step pathway. • To learn more about a reaction: ▪ Thermodynamics ▪ Kinetics

Chlorination of Methane H H H一CH CI-CI heat or light H-H-CI H-CI H H methane chlorine chloromethane hydrogen (methyl chloride) chloride Copyright2010 Pearson Prentice Hall,Inc. Requires heat or light for initiation. The most effective wavelength is blue,which is absorbed by chlorine gas. Many molecules of product are formed from absorption of only one photon of light (chain reaction). Chapter 4 3
Chapter 4 3 Chlorination of Methane • Requires heat or light for initiation. • The most effective wavelength is blue, which is absorbed by chlorine gas. • Many molecules of product are formed from absorption of only one photon of light (chain reaction)

The Free-Radical Chain Reaction Initiation generates a radical intermediate. Propagation:The intermediate reacts with a stable molecule to produce another reactive intermediate(and a product molecule). Termination:Side reactions that destroy the reactive intermediate. Chapter 4 4
Chapter 4 4 The Free-Radical Chain Reaction • Initiation generates a radical intermediate. • Propagation: The intermediate reacts with a stable molecule to produce another reactive intermediate (and a product molecule). • Termination: Side reactions that destroy the reactive intermediate

Initiation Step:Formation of Chlorine Atom photon (hv) :g+ Copyright2010 Pearson Prentice Hall,Inc. A chlorine molecule splits homolytically into chlorine atoms (free radicals). Chapter4 5
Chapter 4 5 Initiation Step: Formation of Chlorine Atom A chlorine molecule splits homolytically into chlorine atoms (free radicals)
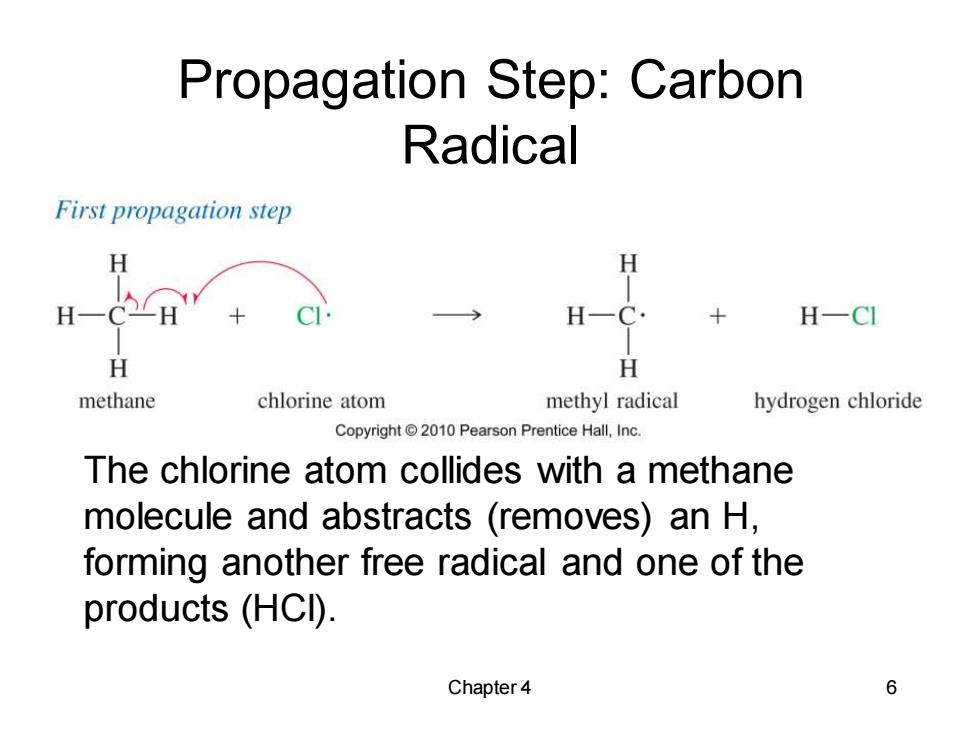
Propagation Step:Carbon Radical First propagation step H CH H-CI H H methane chlorine atom methyl radical hydrogen chloride Copyright2010 Pearson Prentice Hall,Inc. The chlorine atom collides with a methane molecule and abstracts (removes)an H, forming another free radical and one of the products (HCI). Chapter 4 6
Chapter 4 6 Propagation Step: Carbon Radical The chlorine atom collides with a methane molecule and abstracts (removes) an H, forming another free radical and one of the products (HCl)
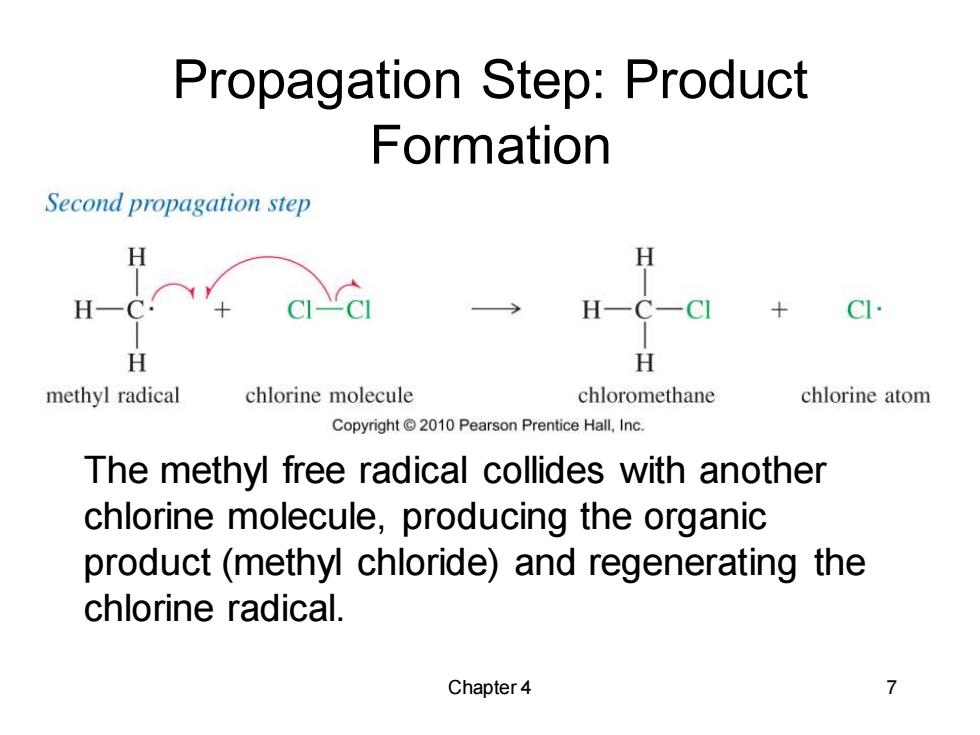
Propagation Step:Product Formation Second propagation step H H H- C-CI H H methyl radical chlorine molecule chloromethane chlorine atom Copyright2010 Pearson Prentice Hall,Inc. The methyl free radical collides with another chlorine molecule,producing the organic product(methyl chloride)and regenerating the chlorine radical. Chapter 4 7
Chapter 4 7 Propagation Step: Product Formation The methyl free radical collides with another chlorine molecule, producing the organic product (methyl chloride) and regenerating the chlorine radical
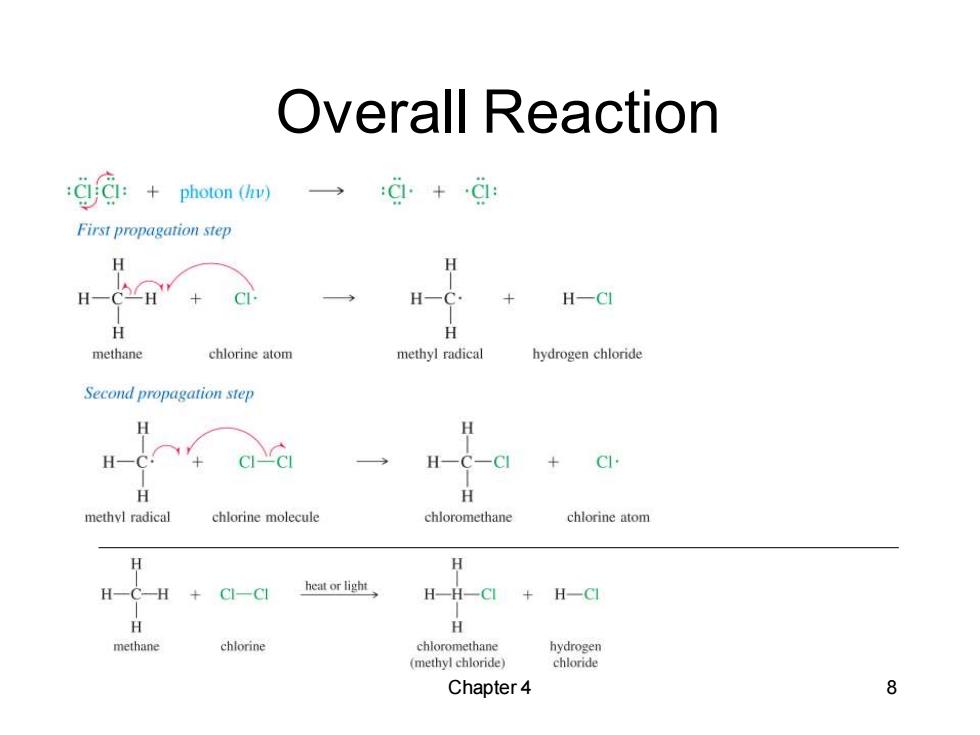
Overall Reaction photon ( :C+C1: First propagation step H H H- H H一C H一CI H H methane chlorine atom methyl radical hydrogen chloride Second propagation step H H H-C. H一C-C CI methyl radical chlorine molecule chloromethane chlorine atom H H HCH+C一CI heat or light H-H-CI+H一C H H methane chlorine chloromethane hydrogen (methyl chloride) chloride Chapter 4 8
Chapter 4 8 Overall Reaction
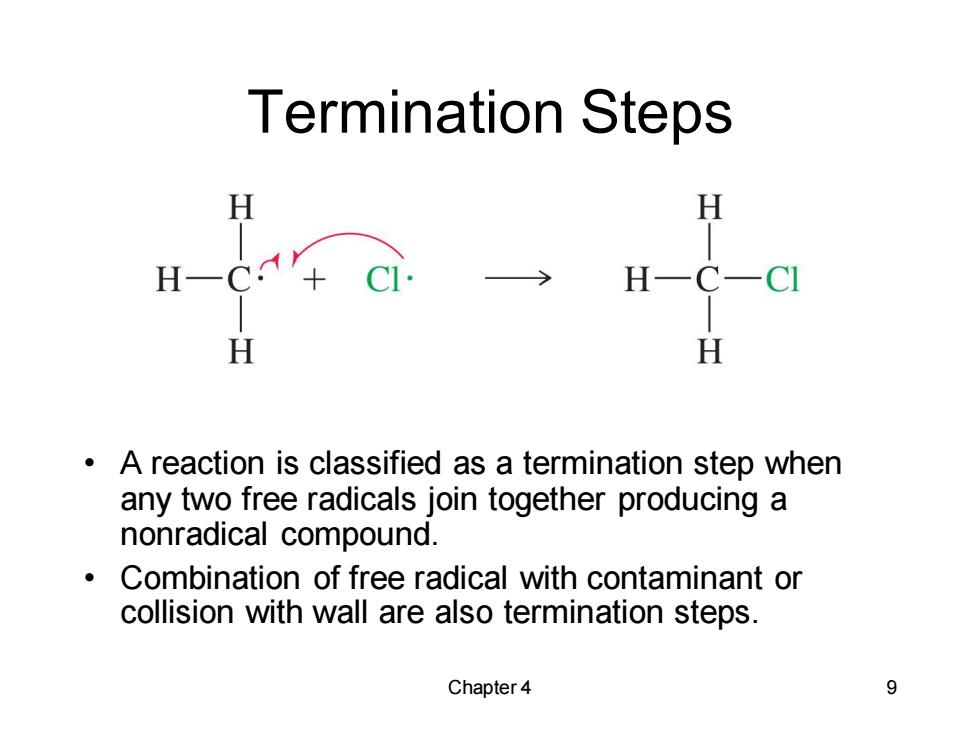
Termination Steps H H H H H A reaction is classified as a termination step when any two free radicals join together producing a nonradical compound. Combination of free radical with contaminant or collision with wall are also termination steps. Chapter4 9
Chapter 4 9 Termination Steps • A reaction is classified as a termination step when any two free radicals join together producing a nonradical compound. • Combination of free radical with contaminant or collision with wall are also termination steps

More Termination Steps H一( C H CI-CI H HH ·CH H一C一C一H H H HH H H H collides with wall wall H H Cl·collides with wall wall Copyright 2010 Pearson Prentice Hall,Inc Chapter 4 10
Chapter 4 10 More Termination Steps