
Organic Chemistry,5th Edition L.G.Wade,Jr. Chapter 13 Nuclear Magnetic Resonance Spectroscopy Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2003,Prentice Hall
Chapter 13 Nuclear Magnetic Resonance Spectroscopy Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2003, Prentice Hall Organic Chemistry, 5th Edition L. G. Wade, Jr
Introduction NMR is the most powerful tool available for organic structure determination. It is used to study a wide variety of nuclei: X1H >13C >15N >19F >31P => Chapter 13 2
Chapter 13 2 Introduction • NMR is the most powerful tool available for organic structure determination. • It is used to study a wide variety of nuclei: ➢1H ➢13C ➢15N ➢19F ➢31P =>
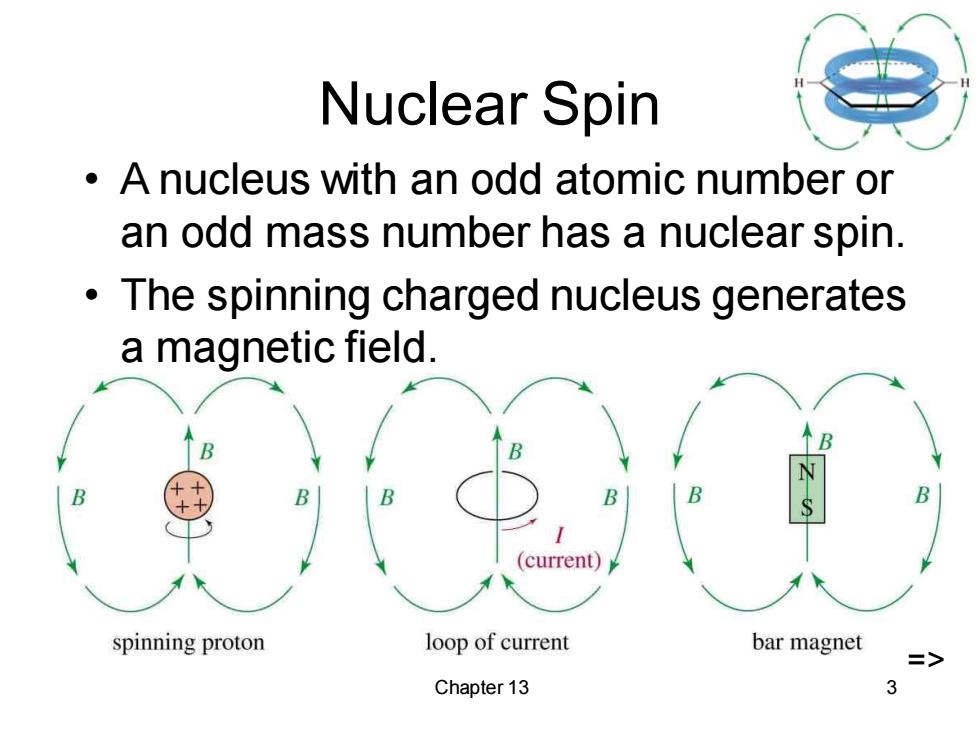
Nuclear Spin A nucleus with an odd atomic number or an odd mass number has a nuclear spin. The spinning charged nucleus generates a magnetic field. (current) spinning proton loop of current bar magnet => Chapter 13 3
Chapter 13 3 Nuclear Spin • A nucleus with an odd atomic number or an odd mass number has a nuclear spin. • The spinning charged nucleus generates a magnetic field. =>
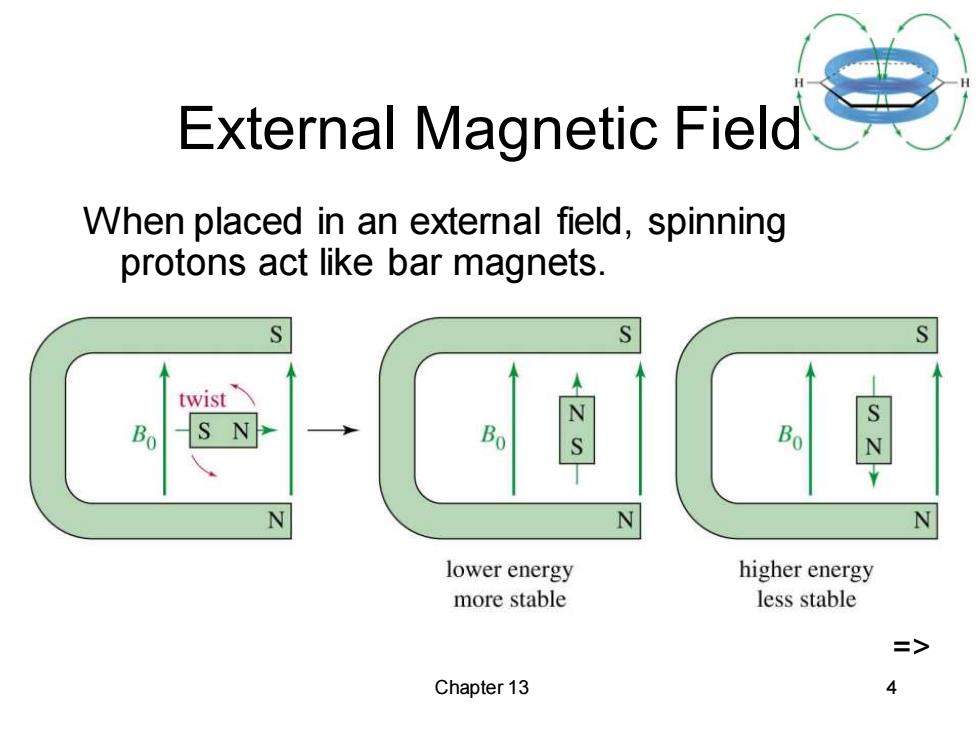
External Magnetic Field When placed in an external field,spinning protons act like bar magnets. S twist N S N 60 Bo N N N N lower energy higher energy more stable less stable 三> Chapter 13 4
Chapter 13 4 External Magnetic Field When placed in an external field, spinning protons act like bar magnets. =>
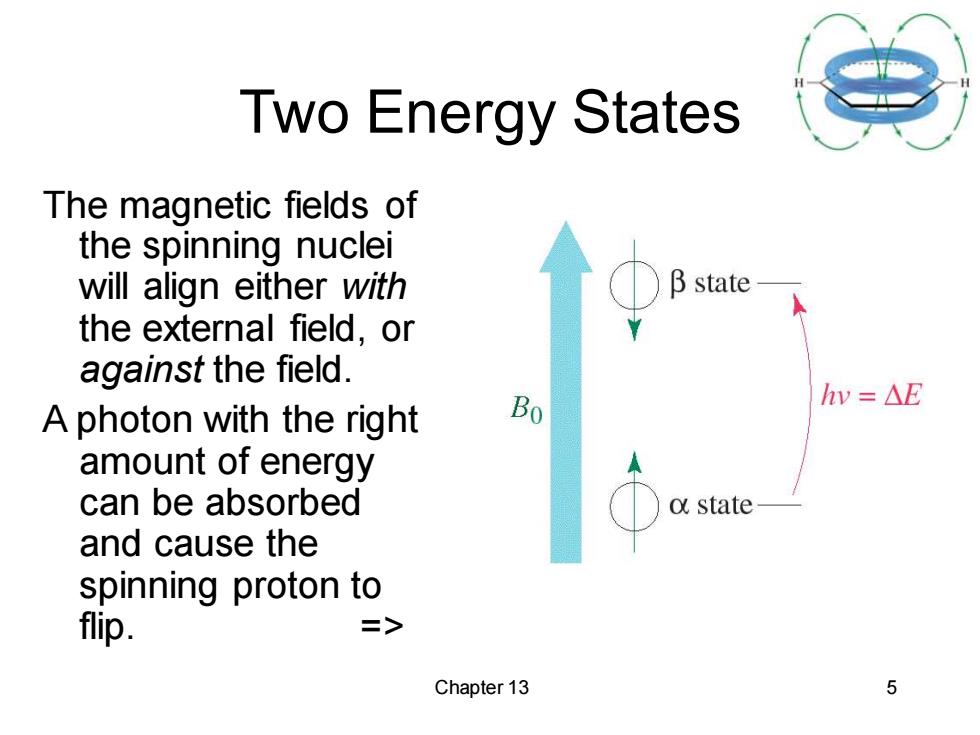
Two Energy States The magnetic fields of the spinning nuclei will align either with B state the external field,or against the field. Bo hv=△E A photon with the right amount of energy can be absorbed o state and cause the spinning proton to flip. => Chapter 13 5
Chapter 13 5 Two Energy States The magnetic fields of the spinning nuclei will align either with the external field, or against the field. A photon with the right amount of energy can be absorbed and cause the spinning proton to flip. =>
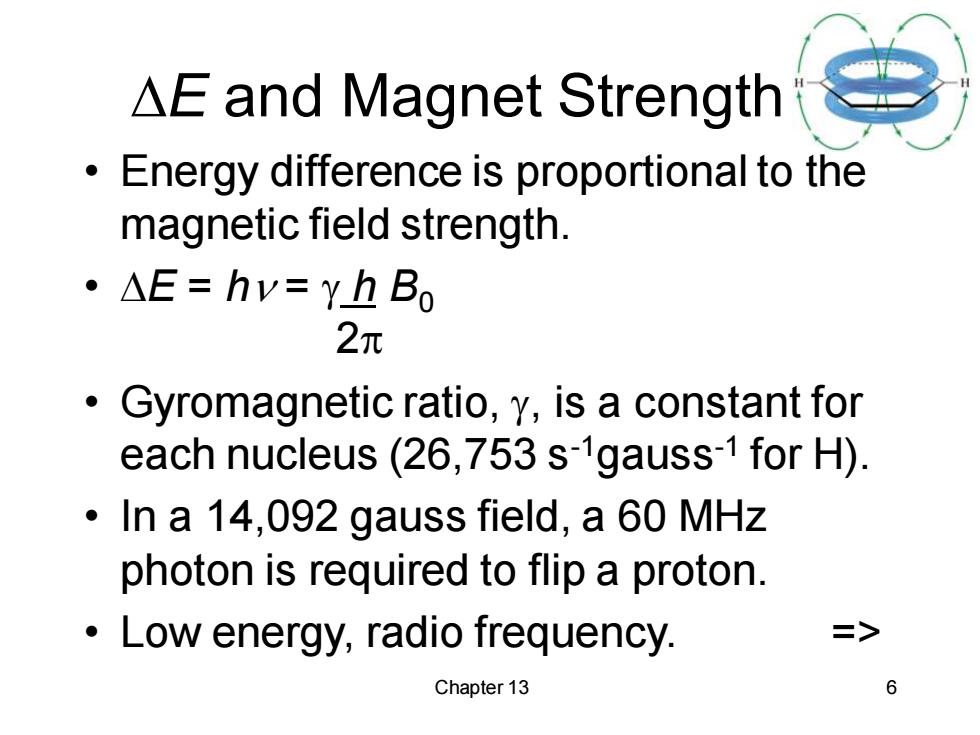
△E and Magnet Strength Energy difference is proportional to the magnetic field strength. 。△E=hv=YhBo 2元 Gyromagnetic ratio,y,is a constant for each nucleus(26,753 s-1gauss-1 for H). In a 14,092 gauss field,a 60 MHz photon is required to flip a proton. Low energy,radio frequency. => Chapter 13 6
Chapter 13 6 E and Magnet Strength • Energy difference is proportional to the magnetic field strength. • E = h = h B0 2 • Gyromagnetic ratio, , is a constant for each nucleus (26,753 s-1gauss-1 for H). • In a 14,092 gauss field, a 60 MHz photon is required to flip a proton. • Low energy, radio frequency. =>

Magnetic Shielding If all protons absorbed the same amount of energy in a given magnetic field,not much information could be obtained. But protons are surrounded by electrons that shield them from the external field. 。 Circulating electrons create an induced magnetic field that opposes the external magnetic field. => Chapter 13 7
Chapter 13 7 Magnetic Shielding • If all protons absorbed the same amount of energy in a given magnetic field, not much information could be obtained. • But protons are surrounded by electrons that shield them from the external field. • Circulating electrons create an induced magnetic field that opposes the external magnetic field. =>
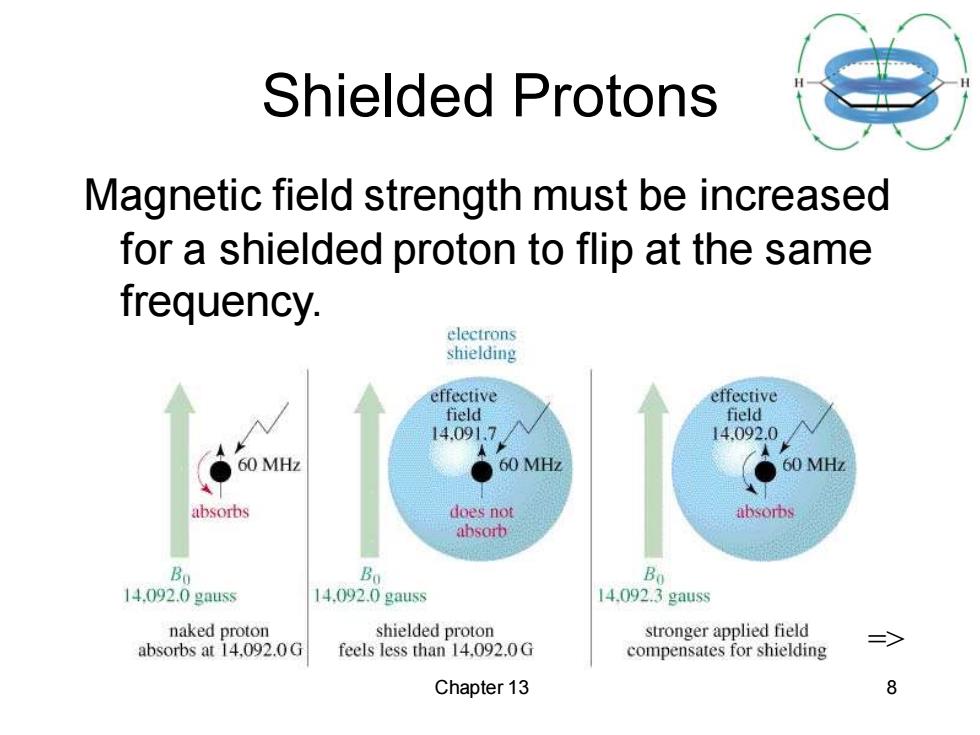
Shielded Protons Magnetic field strength must be increased for a shielded proton to flip at the same frequency. electrons shielding effective effective field field 14.091.7 14.092.0 60 MHz 60MH2 ● 60 MHz absorbs does not absorbs absorb B列 Bo Bo 14.092.0 gauss 14.092.0 gauss 14.092.3 gauss naked proton shielded proton stronger applied field absorbs at 14,092.0G feels less than 14.092.0G compensates for shielding Chapter 13 8
Chapter 13 8 Shielded Protons Magnetic field strength must be increased for a shielded proton to flip at the same frequency. =>
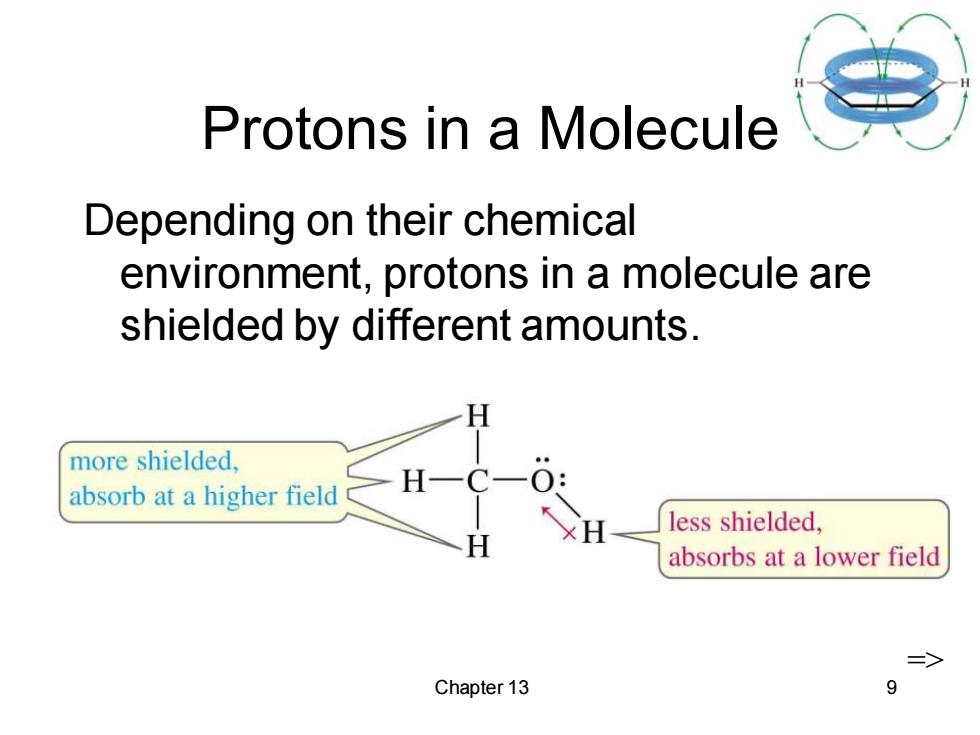
Protons in a Molecule Depending on their chemical environment,protons in a molecule are shielded by different amounts. H more shielded. absorb at a higher field H less shielded, absorbs at a lower field > Chapter 13 9
Chapter 13 9 Protons in a Molecule Depending on their chemical environment, protons in a molecule are shielded by different amounts. =>
NMR Signals The number of signals shows how many different kinds of protons are present. The location of the signals shows how shielded or deshielded the proton is. The intensity of the signal shows the number of protons of that type. Signal splitting shows the number of protons on adjacent atoms. 二> Chapter 13 10
Chapter 13 10 NMR Signals • The number of signals shows how many different kinds of protons are present. • The location of the signals shows how shielded or deshielded the proton is. • The intensity of the signal shows the number of protons of that type. • Signal splitting shows the number of protons on adjacent atoms. =>