
Organic Chemistry,7th Edition L.G.Wade,Jr. Chapter 5 Stereochemistry Copyright 2010 Pearson Education,Inc
Chapter 5 Copyright © 2010 Pearson Education, Inc. Organic Chemistry, 7th Edition L. G. Wade, Jr. Stereochemistry

Chirality right hand left hand ·“Handedness”:Right glove doesn'tfit the left hand. Mirror-image object is different from the original object. Chapter 5 2
Chapter 5 2 Chirality • “Handedness”: Right glove doesn’t fit the left hand. • Mirror-image object is different from the original object
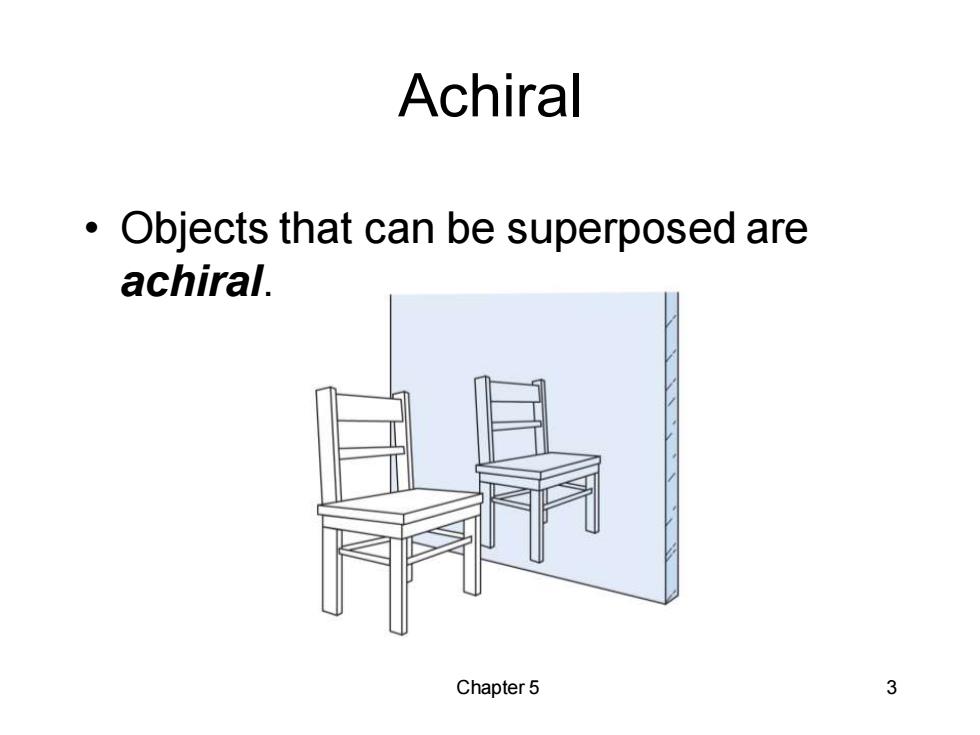
Achiral Objects that can be superposed are achiral. Chapter 5 3
Chapter 5 3 Achiral • Objects that can be superposed are achiral
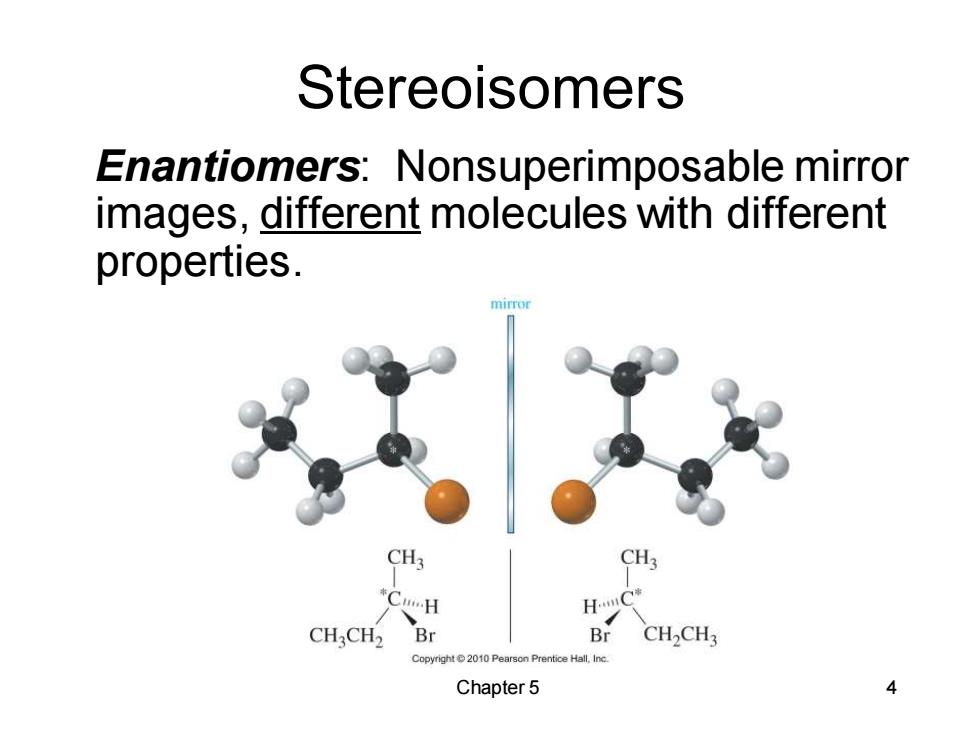
Stereoisomers Enantiomers:Nonsuperimposable mirror images,different molecules with different properties. mirror CH CHCH2 Br Br CH2CH3 Copyright2010 Pearson Prentice Hall.Inc Chapter 5 4
Chapter 5 4 Stereoisomers Enantiomers: Nonsuperimposable mirror images, different molecules with different properties
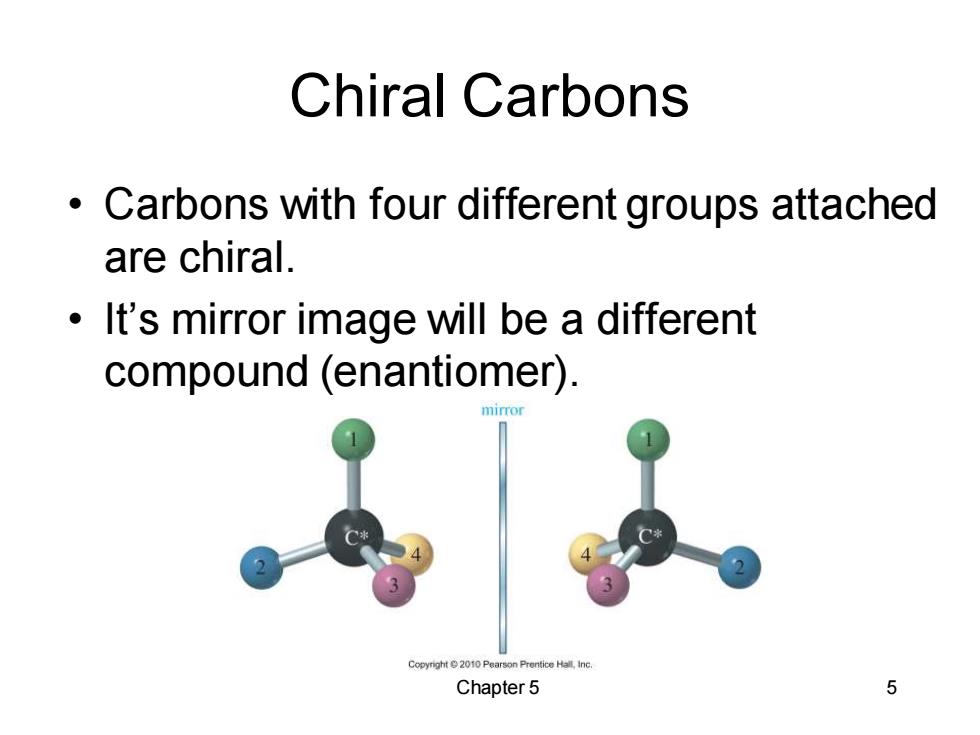
Chiral Carbons Carbons with four different groups attached are chiral. It's mirror image will be a different compound (enantiomer). Copyright 2010 Pearson Prentice Hall,inc. Chapter 5 5
Chapter 5 5 Chiral Carbons • Carbons with four different groups attached are chiral. • It’s mirror image will be a different compound (enantiomer)
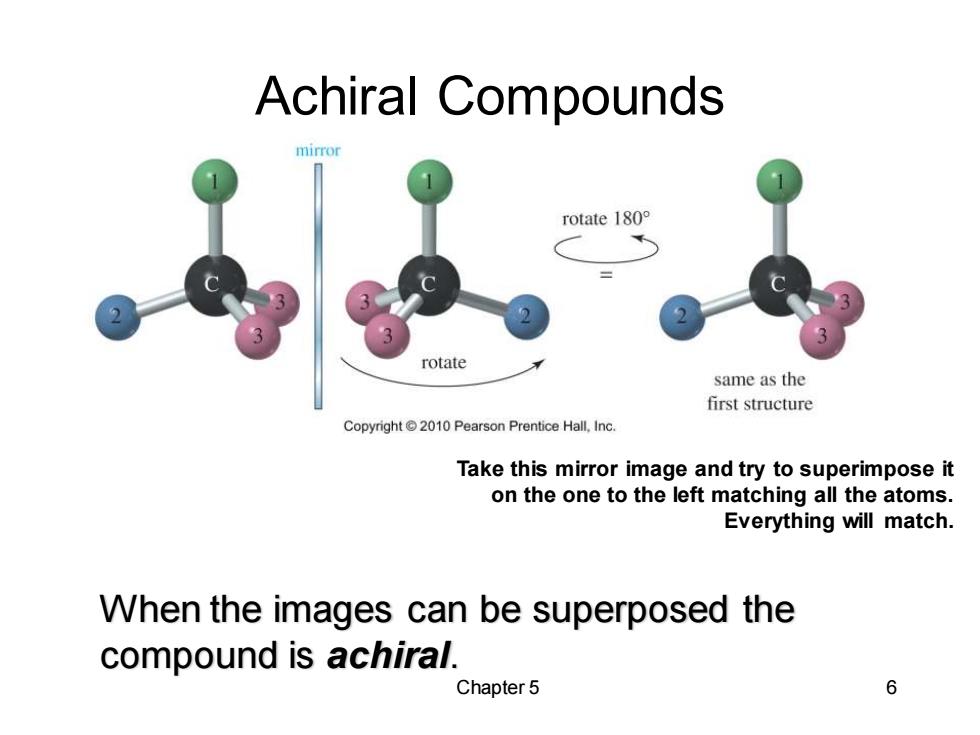
Achiral Compounds mirror rotate 180° rotate same as the first structure Copyright 2010 Pearson Prentice Hall,Inc. Take this mirror image and try to superimpose it on the one to the left matching all the atoms. Everything will match. When the images can be superposed the compound is achiral. Chapter 5 6
Chapter 5 6 Achiral Compounds Take this mirror image and try to superimpose it on the one to the left matching all the atoms. Everything will match. When the images can be superposed the compound is achiral

Planes of Symmetry 3 viewed from this angle Copyright 2010 Pearson Prentice Hall,Inc. A molecule that has a plane of symmetry is achiral. Chapter 5 7
Chapter 5 7 Planes of Symmetry • A molecule that has a plane of symmetry is achiral
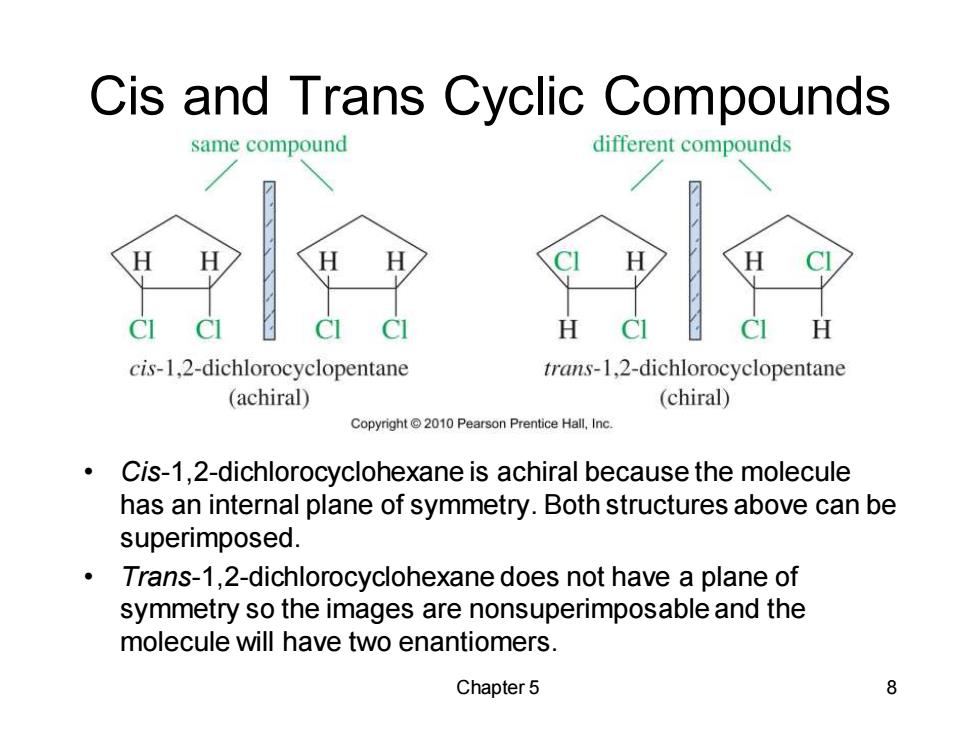
Cis and Trans Cyclic Compounds same compound different compounds ci CI H cis-1,2-dichlorocyclopentane trans-1,2-dichlorocyclopentane (achiral) (chiral) Copyright 2010 Pearson Prentice Hall,Inc. Cis-1,2-dichlorocyclohexane is achiral because the molecule has an internal plane of symmetry.Both structures above can be superimposed. Trans-1,2-dichlorocyclohexane does not have a plane of symmetry so the images are nonsuperimposable and the molecule will have two enantiomers. Chapter 5 8
Chapter 5 8 Cis and Trans Cyclic Compounds • Cis-1,2-dichlorocyclohexane is achiral because the molecule has an internal plane of symmetry. Both structures above can be superimposed. • Trans-1,2-dichlorocyclohexane does not have a plane of symmetry so the images are nonsuperimposable and the molecule will have two enantiomers
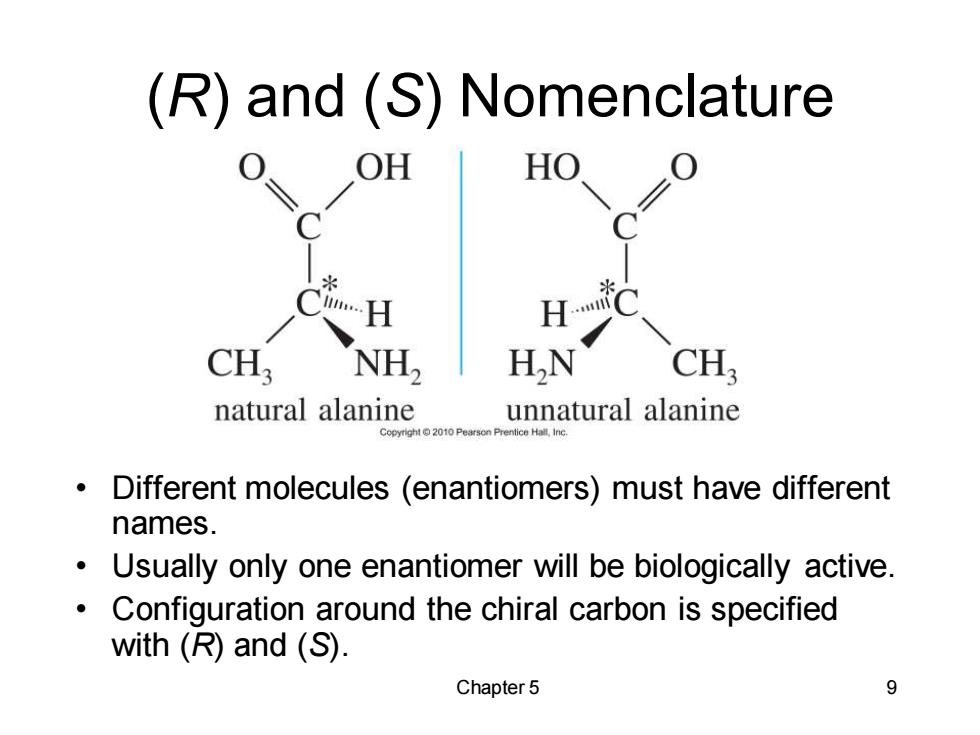
(R)and(S)Nomenclature OH HO CH; NH2 HN CH natural alanine unnatural alanine Copyright2010 Pearson Prentice Hall,Inc Different molecules (enantiomers)must have different names. Usually only one enantiomer will be biologically active. Configuration around the chiral carbon is specified with (R)and (S). Chapter 5 9
Chapter 5 9 (R) and (S) Nomenclature • Different molecules (enantiomers) must have different names. • Usually only one enantiomer will be biologically active. • Configuration around the chiral carbon is specified with (R) and (S)

Cahn-Ingold-Prelog Rules Assign a priority number to each group attached to the chiral carbon. Priority is assigned according to atomic number.The highest atomic number assigned is the highest priority #1. In case of ties,look at the next atoms along the chain. Double and triple bonds are treated like bonds to duplicate atoms. Chapter 5 10
Chapter 5 10 Cahn–Ingold–Prelog Rules • Assign a priority number to each group attached to the chiral carbon. • Priority is assigned according to atomic number. The highest atomic number assigned is the highest priority #1. • In case of ties, look at the next atoms along the chain. • Double and triple bonds are treated like bonds to duplicate atoms