
Organic Chemistry,7th Edition L.G.Wade,Jr. Chapter 12 Infrared Spectroscopy and Mass Spectrometry Copyright 2010 Pearson Education,Inc
Chapter 12 Copyright © 2010 Pearson Education, Inc. Organic Chemistry, 7th Edition L. G. Wade, Jr. Infrared Spectroscopy and Mass Spectrometry

Introduction ● Spectroscopy is a technique used to determine the structure of a compound. Most techniques are nondestructive (it destroys little or no sample). Absorption spectroscopy measures the amount of light absorbed by the sample as a function of wavelength. Chapter 12 2
Chapter 12 2 Introduction • Spectroscopy is a technique used to determine the structure of a compound. • Most techniques are nondestructive (it destroys little or no sample). • Absorption spectroscopy measures the amount of light absorbed by the sample as a function of wavelength
Types of Spectroscopy Infrared(IR)spectroscopy measures the bond vibration frequencies in a molecule and is used to determine the functional group. Mass spectrometry (MS)fragments the molecule and measures their mass.MS can give the molecular weight of the compound and functional groups. Nuclear magnetic resonance (NMR)spectroscopy analyzes the environment of the hydrogens in a compound.This gives useful clues as to the alkyl and other functional groups present. Ultraviolet (UV)spectroscopy uses electronic transitions to determine bonding patterns. Chapter 12 3
Chapter 12 3 Types of Spectroscopy • Infrared (IR) spectroscopy measures the bond vibration frequencies in a molecule and is used to determine the functional group. • Mass spectrometry (MS) fragments the molecule and measures their mass. MS can give the molecular weight of the compound and functional groups. • Nuclear magnetic resonance (NMR) spectroscopy analyzes the environment of the hydrogens in a compound. This gives useful clues as to the alkyl and other functional groups present. • Ultraviolet (UV) spectroscopy uses electronic transitions to determine bonding patterns
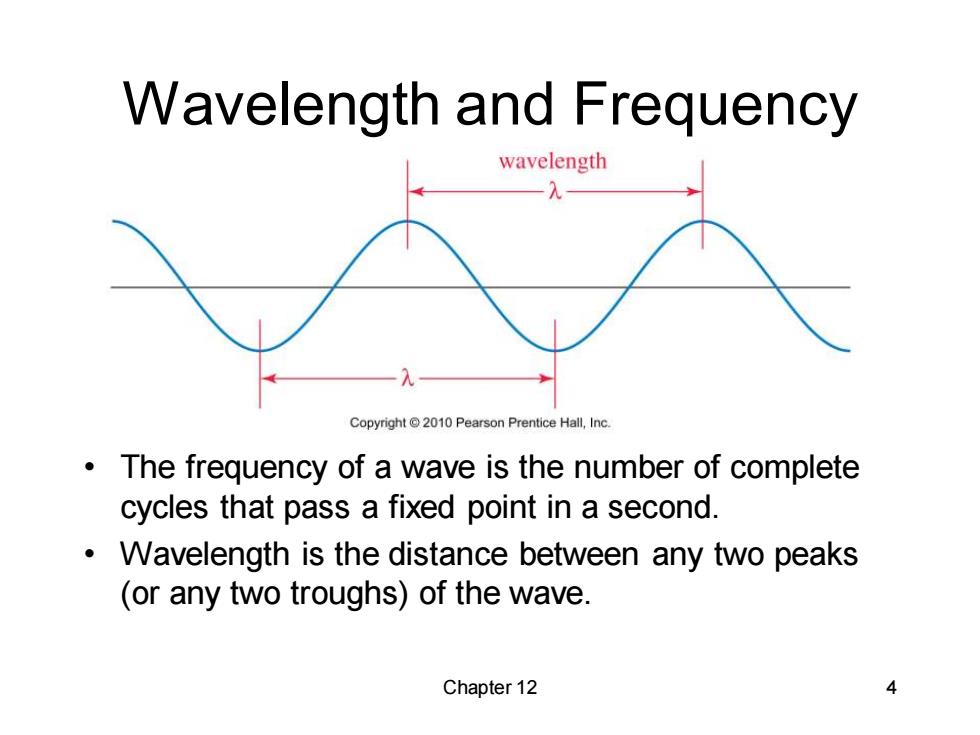
Wavelength and Frequency wavelength Copyright2010 Pearson Prentice Hall,Inc. The frequency of a wave is the number of complete cycles that pass a fixed point in a second. Wavelength is the distance between any two peaks (or any two troughs)of the wave. Chapter 12 4
Chapter 12 4 Wavelength and Frequency • The frequency of a wave is the number of complete cycles that pass a fixed point in a second. • Wavelength is the distance between any two peaks (or any two troughs) of the wave
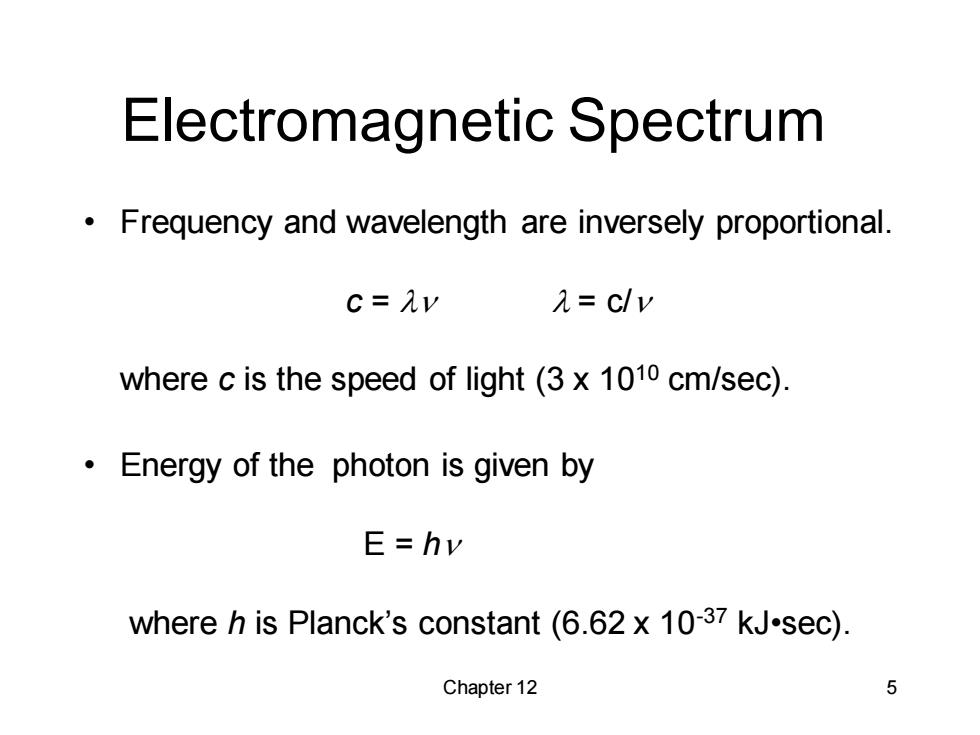
Electromagnetic Spectrum Frequency and wavelength are inversely proportional. c=Av 1=c/v where c is the speed of light (3 x 1010 cm/sec). Energy of the photon is given by E=hv where h is Planck's constant (6.62 x 10-37 kJ.sec). Chapter 12 5
Chapter 12 5 Electromagnetic Spectrum • Frequency and wavelength are inversely proportional. c = ln l = c/n where c is the speed of light (3 x 1010 cm/sec). • Energy of the photon is given by E = hn where h is Planck’s constant (6.62 x 10-37 kJ•sec)

The Electromagnetic Spectrum Wavelength (A) Region Energy Molecular effects higher frequency cm kJ/mol shorter wavelength 109 gamma rays 107 10-7 X rays 105 ionization vacuum UV 103 10-5 near UV electronic transitions 10-4 visible 102 infrared 10-3 10 molecular vibrations (R) 1 10-1 microwave rotational motion 10-2 102 lower frequency radio 10-4 104 longer wavelength 10-6 nuclear spin transitions Copyright 2010 Pearson Prentice Hall,Inc. Chapter 12 6
Chapter 12 6 The Electromagnetic Spectrum

The IR Region From right below the visible region to just above the highest microwave and radar frequencies. Wavelengths are usually 2.5 x 10-4 to 25 x 104 cm. More common units are wavenumbers, or cm-1,the reciprocal of the wavelength in centimeters. Wavenumbers are proportional to frequency and energy. Chapter 12 7
Chapter 12 7 The IR Region • From right below the visible region to just above the highest microwave and radar frequencies . • Wavelengths are usually 2.5 x 10-4 to 25 x 10-4 cm. • More common units are wavenumbers, or cm-1 , the reciprocal of the wavelength in centimeters. • Wavenumbers are proportional to frequency and energy
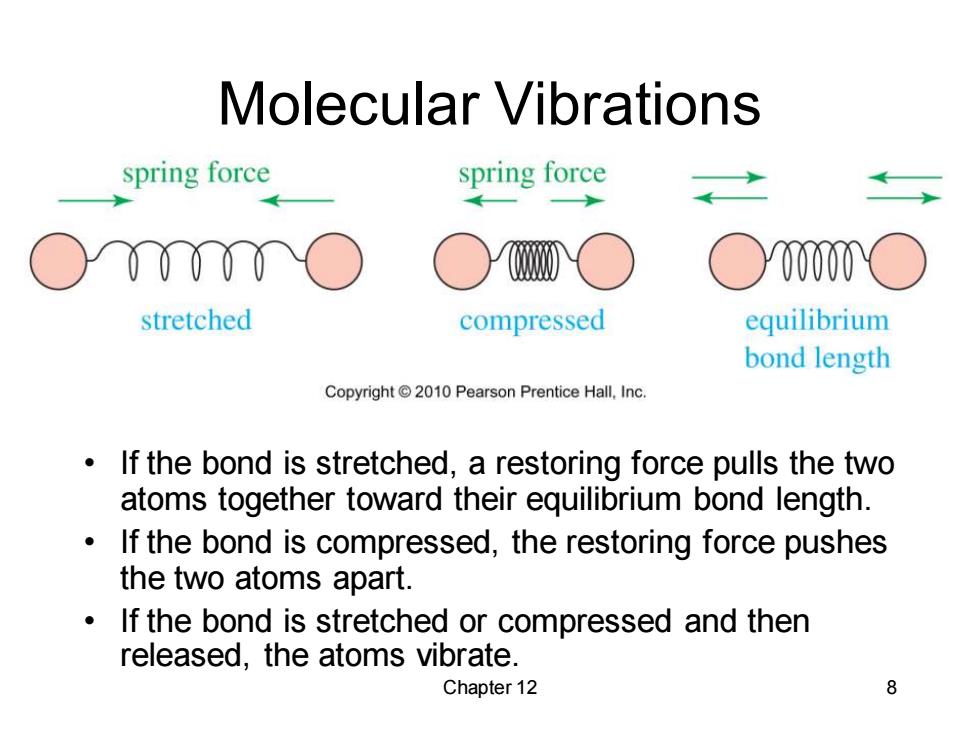
Molecular Vibrations spring force spring force ○00000 stretched compressed equilibrium bond length Copyright 2010 Pearson Prentice Hall,Inc. If the bond is stretched,a restoring force pulls the two atoms together toward their equilibrium bond length. If the bond is compressed,the restoring force pushes the two atoms apart. If the bond is stretched or compressed and then released,the atoms vibrate. Chapter 12 8
Chapter 12 8 Molecular Vibrations • If the bond is stretched, a restoring force pulls the two atoms together toward their equilibrium bond length. • If the bond is compressed, the restoring force pushes the two atoms apart. • If the bond is stretched or compressed and then released, the atoms vibrate

Stretching Frequencies TABLE 12-1 Bond Stretching Frequencies In a group of bonds with similar bond energies,the frequency decreases with increasing atomic weight.In a group of bonds between similar atoms,the frequency increases with bond energy. The bond energies and frequencies listed hereare approximate. Bond Bond Energy [kJ(kcal)] Stretching Frequency(cm) Frequency decreases with increasing atomic mass C一H 420(100) 3000 C-D heavier atoms 420(100) 2100 y decreases C-C 350(83) 1200 Frequency increases with bond energy C-C 350(83) 1200 C=C 611(146) stronger 1660 v increases C=C bond 840(200) 2200 C-N 305(73) 1200 C=N 615(147) 1650 C=N 891(213) 2200 C-0 360(86) 1100 C=0 745(178) 1700 Copyright2010 Pearson Prentice Hall.Inc. Frequency decreases with increasing atomic mass. Frequency increases with increasing bond energy. Chapter 12 9
Chapter 12 9 Stretching Frequencies • Frequency decreases with increasing atomic mass. • Frequency increases with increasing bond energy
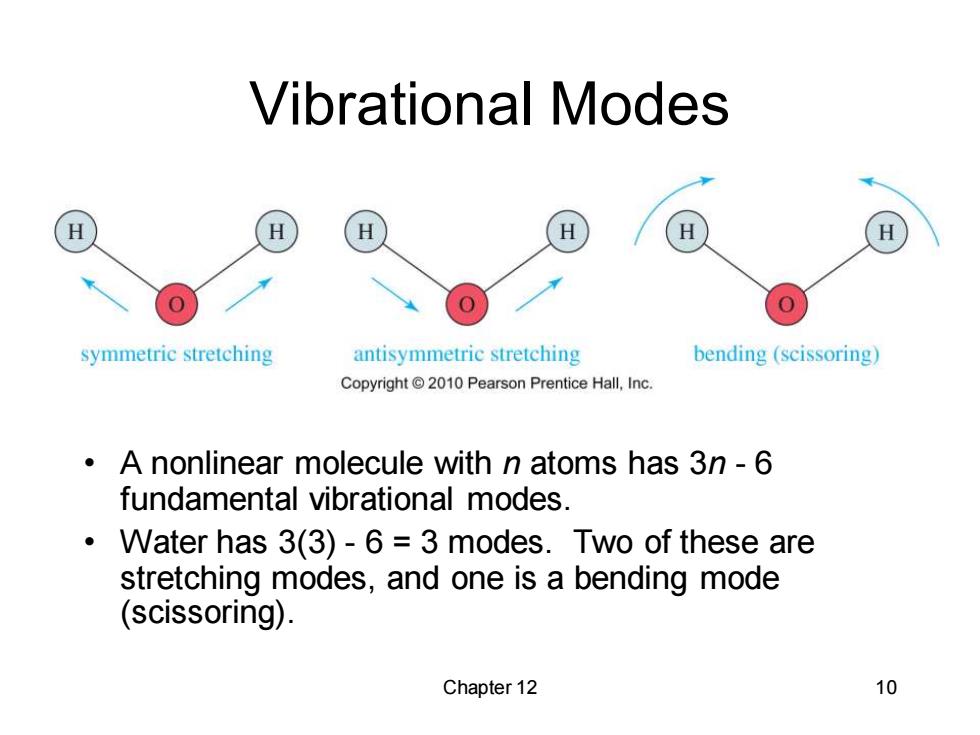
Vibrational Modes H symmetric stretching antisymmetric stretching bending(scissoring) Copyright2010 Pearson Prentice Hall,Inc A nonlinear molecule with n atoms has 3n-6 fundamental vibrational modes. Water has 3(3)-6 3 modes.Two of these are stretching modes,and one is a bending mode (scissoring). Chapter 12 10
Chapter 12 10 Vibrational Modes • A nonlinear molecule with n atoms has 3n - 6 fundamental vibrational modes. • Water has 3(3) - 6 = 3 modes. Two of these are stretching modes, and one is a bending mode (scissoring)