
Organic Chemistry,7th Edition L.G.Wade,Jr. Chapter 18 Ketones and Aldehydes Copyright 2010 Pearson Education,Inc
Chapter 18 Copyright © 2010 Pearson Education, Inc. Organic Chemistry, 7th Edition L. G. Wade, Jr. Ketones and Aldehydes
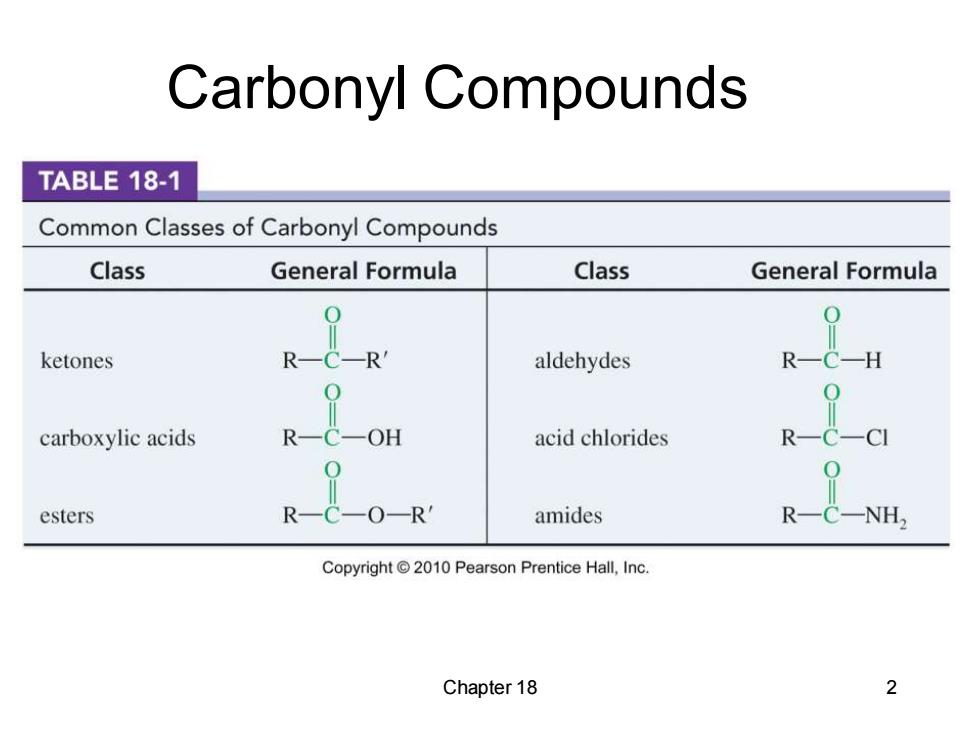
Carbonyl Compounds TABLE 18-1 Common Classes of Carbonyl Compounds Class General Formula Class General Formula ketones R-C-R aldehydes R-C-H carboxylic acids C-OH acid chlorides R-C-CI esters R-C-O-R amides R NH Copyright2010 Pearson Prentice Hall,Inc. Chapter 18 2
Chapter 18 2 Carbonyl Compounds

Carbonyl Structure length energy ketone C=O bond 1.23A 745 kJ/mol (178 kcal/mol alkene C=C bond 1.34A 611 kJ/mol (146 kcal/mol) Copyright 2010 Pearson Prentice Hall,Inc. Carbon is sp2 hybridized. C-O bond is shorter,stronger,and more polar than C=C bond in alkenes. Chapter 18 3
Chapter 18 3 Carbonyl Structure ▪ Carbon is sp2 hybridized. ▪ C═O bond is shorter, stronger, and more polar than C═C bond in alkenes
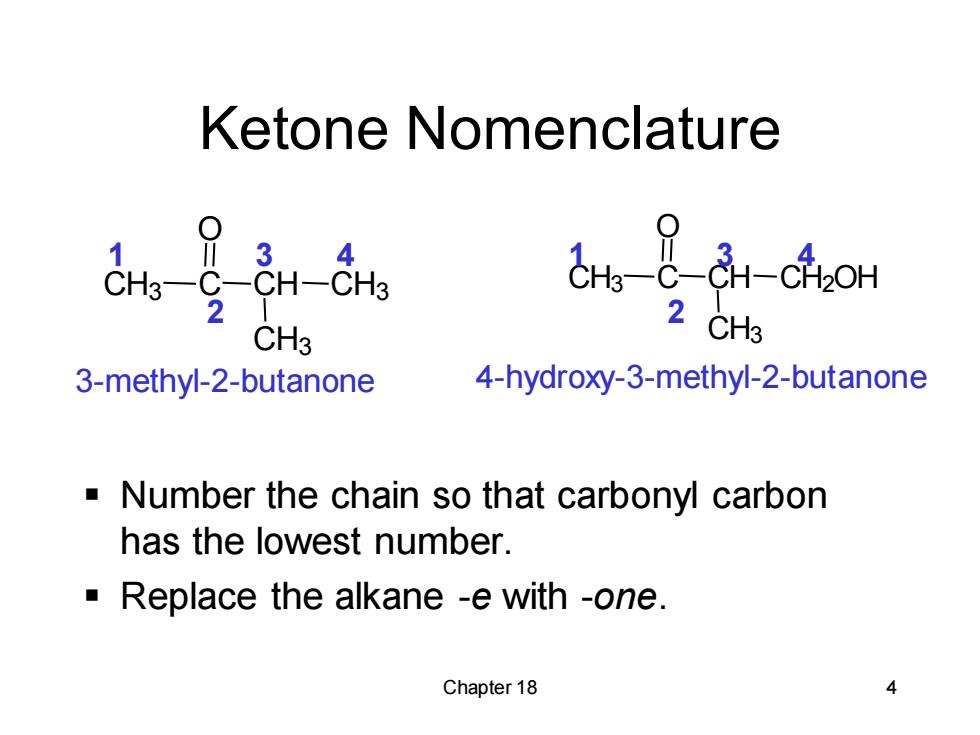
Ketone Nomenclature 3 4 in-chom 2 2 CH3 CH3 3-methyl-2-butanone 4-hydroxy-3-methyl-2-butanone -Number the chain so that carbonyl carbon has the lowest number. Replace the alkane -e with -one. Chapter 18 4
Chapter 18 4 Ketone Nomenclature ▪ Number the chain so that carbonyl carbon has the lowest number. ▪ Replace the alkane -e with -one. 3-methyl-2-butanone 4-hydroxy-3-methyl-2-butanone CH3 C O CH CH3 CH3 CH3 C O CH CH3 CH2OH 1 2 3 4 1 2 3 4
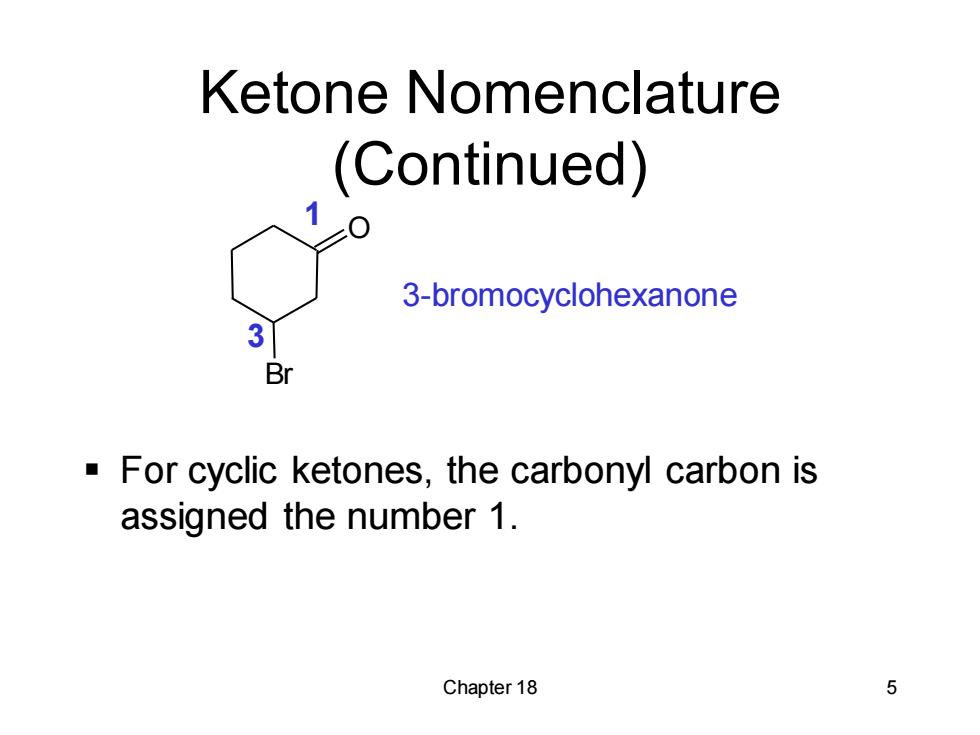
Ketone Nomenclature (Continued) 3-bromocyclohexanone 3 Br For cyclic ketones,the carbonyl carbon is assigned the number 1. Chapter 18 5
Chapter 18 5 Ketone Nomenclature (Continued) ▪ For cyclic ketones, the carbonyl carbon is assigned the number 1. 3-bromocyclohexanone O Br 1 3
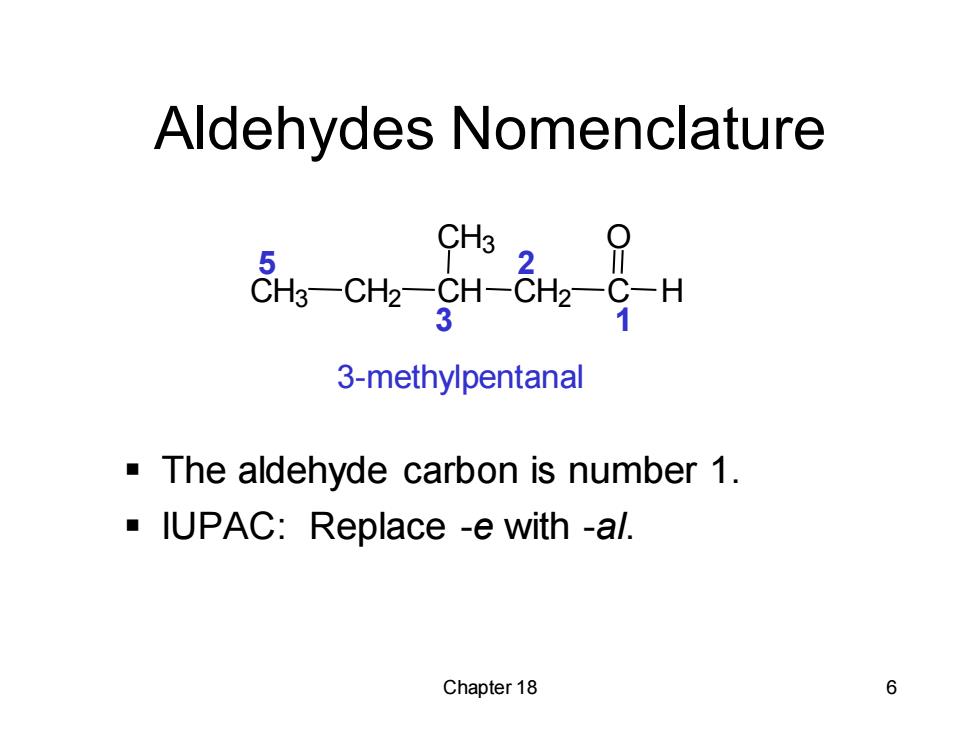
Aldehydes Nomenclature 3 3-methylpentanal The aldehyde carbon is number 1. IUPAC:Replace -e with -al. Chapter 18 6
Chapter 18 6 CH3 CH2 CH CH3 CH2 C H O Aldehydes Nomenclature ▪ The aldehyde carbon is number 1. ▪ IUPAC: Replace -e with -al. 3-methylpentanal 1 2 3 5
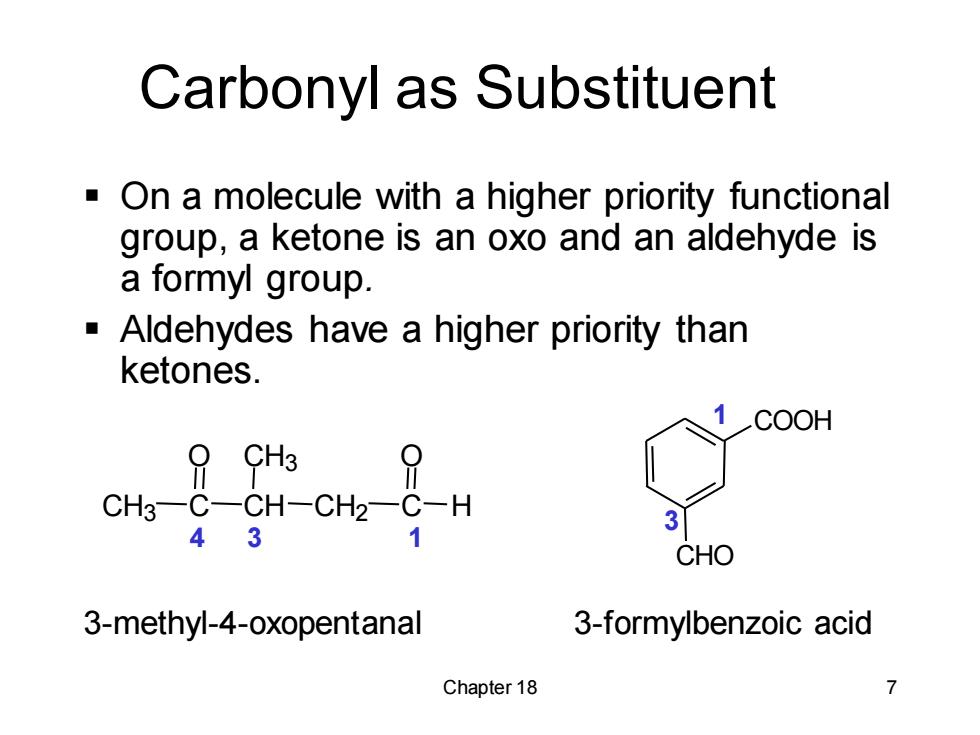
Carbonyl as Substituent -On a molecule with a higher priority functional group,a ketone is an oxo and an aldehyde is a formyl group. Aldehydes have a higher priority than ketones. COOH 9 CH3 CH3-C-CH-CH2-C-H 43 CHO 3-methyl-4-oxopentanal 3-formylbenzoic acid Chapter 18
Chapter 18 7 Carbonyl as Substituent ▪ On a molecule with a higher priority functional group, a ketone is an oxo and an aldehyde is a formyl group. ▪ Aldehydes have a higher priority than ketones. 3-methyl-4-oxopentanal 3-formylbenzoic acid 4 3 1 1 3 CH3 C CH CH3 CH2 C H O O COOH CHO

Common Names for Ketones Named as alkyl attachments to-C=O. Use Greek letters instead of numbers. O CH3-C-CH-CH3 CH3CH-C-CH-CH3 CH3 Br CH3 methyl isopropyl ketone a-bromoethyl isopropyl ketone Chapter 18 8
Chapter 18 8 Common Names for Ketones ▪ Named as alkyl attachments to —C═O. ▪ Use Greek letters instead of numbers. methyl isopropyl ketone a-bromoethyl isopropyl ketone CH3 C O CH CH3 CH3 CH3CH C O CH CH3 CH3 Br
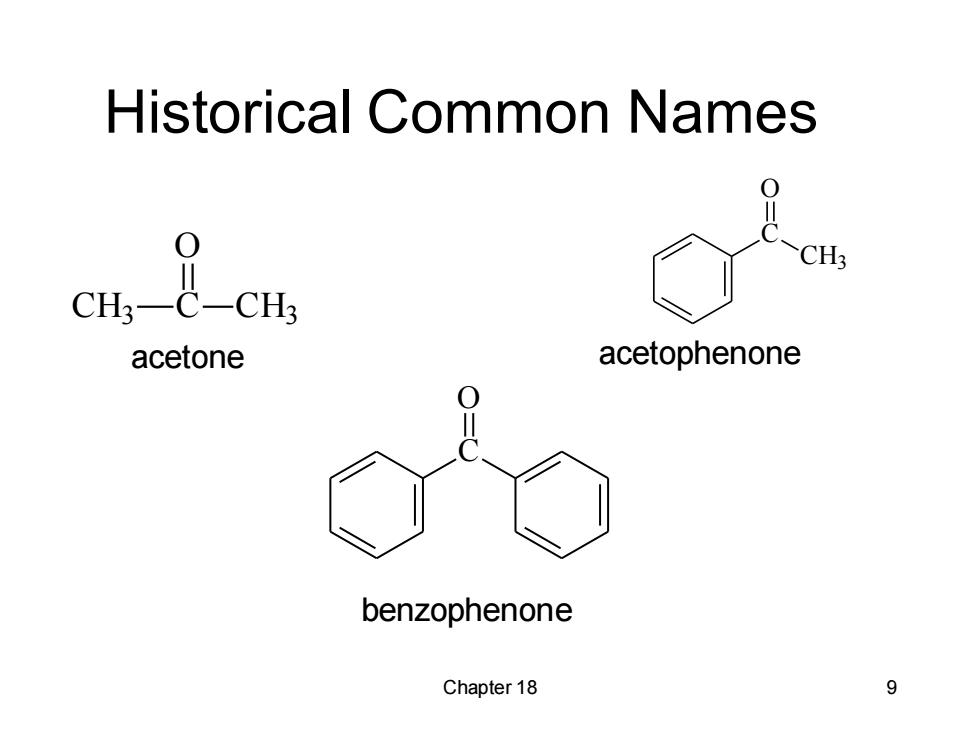
Historical Common Names 0 >CH; acetone acetophenone benzophenone Chapter 18 9
Chapter 18 9 Historical Common Names CH3 C O CH3 C CH3 O C O acetone acetophenone benzophenone
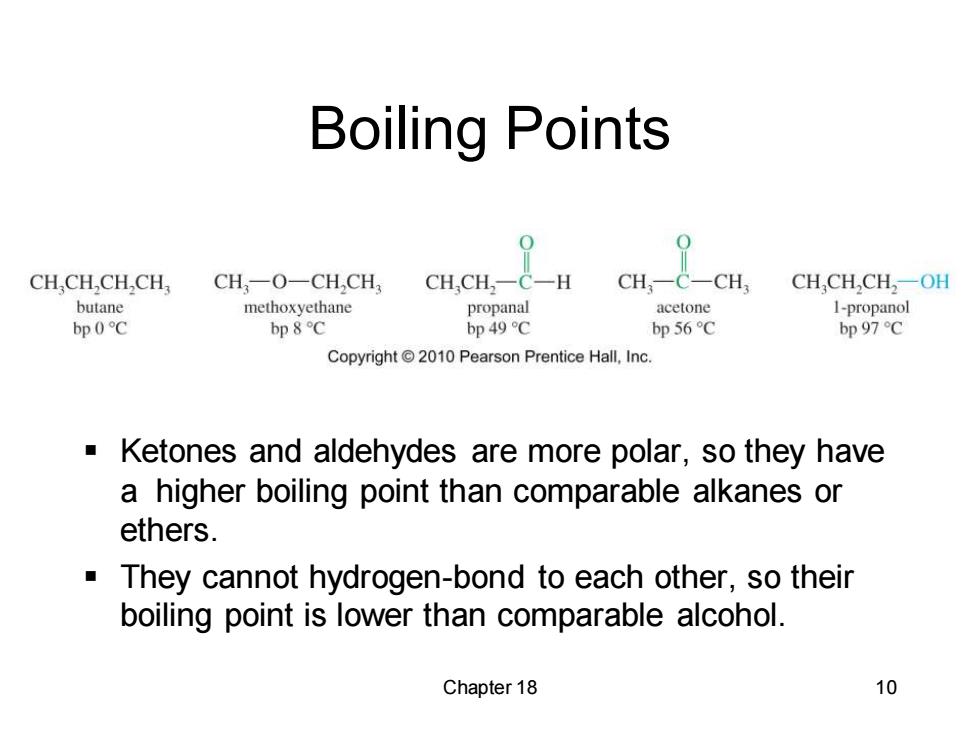
Boiling Points CH.CH,CH,CH, CH一O一CHCH CH,CH2一C一H CH,- C一CH CH,CH,CH2一OH butane methoxyethane propanal acetone I-propanol bp0℃ bp 8C bp 49 C bp 56C bp 97C Copyright2010 Pearson Prentice Hall,Inc. Ketones and aldehydes are more polar,so they have a higher boiling point than comparable alkanes or ethers. They cannot hydrogen-bond to each other,so their boiling point is lower than comparable alcohol. Chapter 18 10
Chapter 18 10 Boiling Points ▪ Ketones and aldehydes are more polar, so they have a higher boiling point than comparable alkanes or ethers. ▪ They cannot hydrogen-bond to each other, so their boiling point is lower than comparable alcohol