
Organic Chemistry,7th Edition L.G.Wade,Jr. Chapter 23 Carbohydrates and Nucleic Acids Copyright 2010 Pearson Education,Inc
Chapter 23 Copyright © 2010 Pearson Education, Inc. Organic Chemistry, 7th Edition L. G. Wade, Jr. Carbohydrates and Nucleic Acids

Carbohydrates Synthesized by plants using sunlight to convert CO2 and H2O to glucose and O2. Polymers include starch and cellulose. -Starch is a storage unit for solar energy. Most sugars have formula C(H2O)n, “hydrate of carbon." Chapter 23 2
Chapter 23 2 Carbohydrates ▪ Synthesized by plants using sunlight to convert CO2 and H2O to glucose and O2 . ▪ Polymers include starch and cellulose. ▪ Starch is a storage unit for solar energy. ▪ Most sugars have formula Cn (H2O)n , “hydrate of carbon

Classification of Carbohydrates Monosaccharides or simple sugars: polyhydroxyaldehydes or aldoses polyhydroxyketones or ketoses Disaccharides can be hydrolyzed to two monosaccharides. Polysaccharides hydrolyze to many monosaccharide units.For example, starch and cellulose have 1000 glucose units. Chapter 23 3
Chapter 23 3 Classification of Carbohydrates ▪ Monosaccharides or simple sugars: ▪ polyhydroxyaldehydes or aldoses ▪ polyhydroxyketones or ketoses ▪ Disaccharides can be hydrolyzed to two monosaccharides. ▪ Polysaccharides hydrolyze to many monosaccharide units. For example, starch and cellulose have > 1000 glucose units

Monosaccharides CHO CH,OH CHOH 2C=0 CHOH CHOH CHO CH,OH CHOH CHOH CHOH 2C=0 SCHOH SCHOH CHOH CHOH CH,OH CH,OH CH,OH CH,OH an aldohexose a ketohexose an aldotetrose a ketotetrose Copyright 2010 Pearson Prentice Hall,Inc. Classified using three criteria: If it contains a ketone or an aldehyde group. Number of carbons in the chain. Configuration of the asymmetric carbon farthest from the carbonyl group. Chapter 23 4
Chapter 23 4 Monosaccharides ▪ Classified using three criteria: ▪ If it contains a ketone or an aldehyde group. ▪ Number of carbons in the chain. ▪ Configuration of the asymmetric carbon farthest from the carbonyl group

(+)and (-)-Glyceraldehydes CHO CHO H-C✉OH HO-CH CH,OH CH,OH (+)-glyceraldehyde (-)-glyceraldehyde D series of sugars L series of sugars Copyright 2010 Pearson Prentice Hall,Inc The (+)enantiomer of glyceraldehyde has its OH group on the right of the Fischer projection. The (-)enantiomer of glyceraldehyde has its OH group on the left of the Fischer projection. Chapter 23 5
Chapter 23 5 (+) and (-)-Glyceraldehydes ▪ The (+) enantiomer of glyceraldehyde has its OH group on the right of the Fischer projection. ▪ The (-) enantiomer of glyceraldehyde has its OH group on the left of the Fischer projection

Degradation of D and L Sugars CHO C02↑ H一C一OH CHO CO2t HO-C-H degrade HO一C一H degrade CHO CO2 H 一OH H一C一OH H-C-OH CHO H-C-OH H-C-OH H-C-OH degrade H-C-OH CH2OH CH2OH CH2OH CH2OH D-(+)-glucose D-(-)-arabinose D-(-)-erythrose D-(+)-glyceraldehyde Copyright2010 Pearson Prentice Hall,Inc. Fischer-Rosanoff Convention D sugars can be degraded to the dextrorotatory (+ form of glyceraldehyde L sugars can be degraded to the levorotatory (-)form of glyceraldehyde. Chapter 23 6
Chapter 23 6 Degradation of D and L Sugars ▪ Fischer–Rosanoff Convention ▪ D sugars can be degraded to the dextrorotatory (+) form of glyceraldehyde. ▪ L sugars can be degraded to the levorotatory (-) form of glyceraldehyde
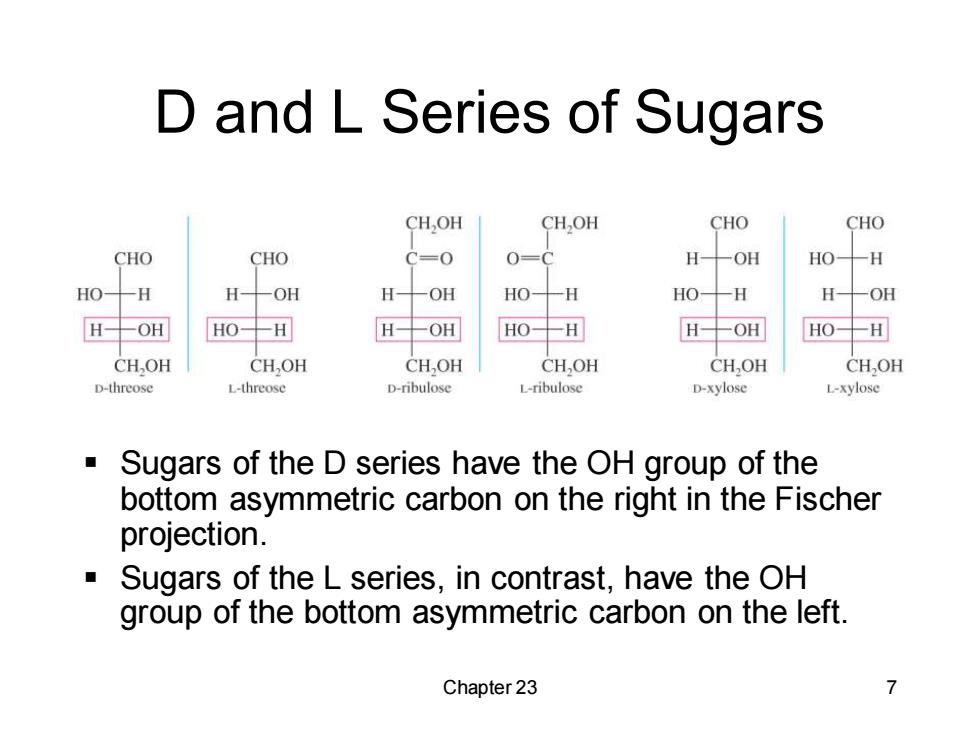
D and L Series of Sugars CHOH CH,OH CHO CHO CHO CHO C=0 0=C H 一OH HO-H HO H -OH H -OH HO 一H HO- -H H -OH H-OH HO-H H-OH HO-H H-OH HO-H CH,OH CHOH CH,OH CHOH CHOH CH,OH D-threose L-threose D-ribulose L-ribulose D-xylose L-xylose Sugars of the D series have the OH group of the bottom asymmetric carbon on the right in the Fischer projection. Sugars of the L series,in contrast,have the OH group of the bottom asymmetric carbon on the left. Chapter 23 7
Chapter 23 7 D and L Series of Sugars ▪ Sugars of the D series have the OH group of the bottom asymmetric carbon on the right in the Fischer projection. ▪ Sugars of the L series, in contrast, have the OH group of the bottom asymmetric carbon on the left
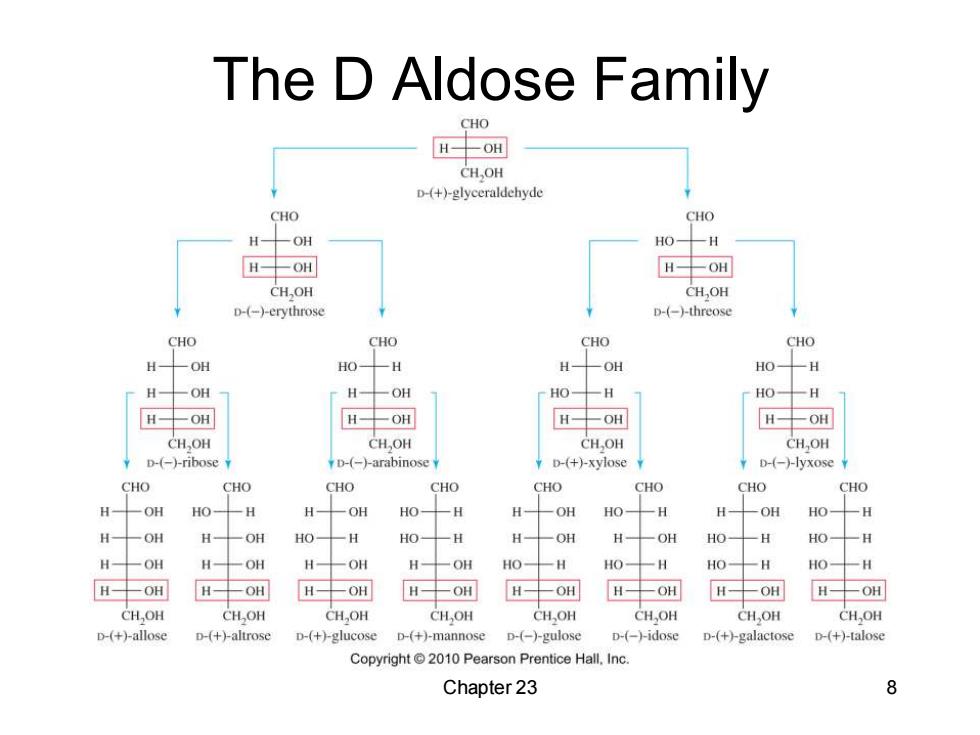
The D Aldose Family CHO H-OH CH.OH D-(+)-glyceraldehyde CHO CHO H一OH HO- 一H H-OH H--OH CH,OH CH,OH D-(-)-erythrose D-(-)-threose CHO CHO CHO CHO H OH HO H一 一OH HO -H H 一OH H- -OH HO- H HO- H H一OH H一OH H-OH H-OH CH,OH CH,OH CHOH CH.OH ◆D-(-ribose ◆D-(--arabinose ¥D-(+)-xylose◆ p-(-)-lyxose CHO CHO CHO CHO CHO CHO CHO CHO H 一OH HO一 -H H-OH HO-H H- 一OH HO- -H H 一OH HO- 一H H 一OH OH HOH HO-H H 一OH H OH HO- -H HO- H HOH H一 一OH H-OH H-OH HO-H HO-H HO- 一H HO-H H-OH H-OH H-OH H-OH HOH H-OH H-OH H-OH CH,OH CHOH CH,OH CH,OH CH,OH CHOH CHOH CH OH D-(+)-allose D-(+)-altrose D-(+)-glucose D-(+)-mannose D-(-)-gulose D-(-→idose D-(+)-galactose D-(+)-talose Copyright 2010 Pearson Prentice Hall.Inc. Chapter 23 8
Chapter 23 8 The D Aldose Family
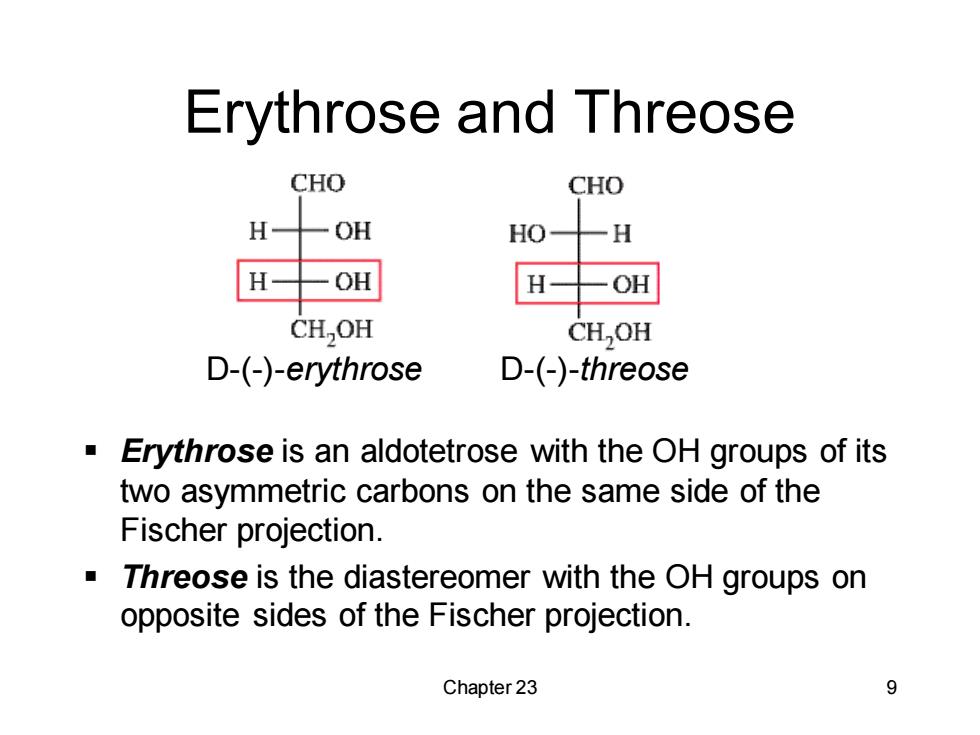
Erythrose and Threose CHO CHO H OH HO H H一OH OH CH,OH CH,OH D-(-)-erythrose D-(-)-threose Erythrose is an aldotetrose with the OH groups of its two asymmetric carbons on the same side of the Fischer projection. Threose is the diastereomer with the OH groups on opposite sides of the Fischer projection. Chapter 23 9
Chapter 23 9 Erythrose and Threose ▪ Erythrose is an aldotetrose with the OH groups of its two asymmetric carbons on the same side of the Fischer projection. ▪ Threose is the diastereomer with the OH groups on opposite sides of the Fischer projection. D-(-)-erythrose D-(-)-threose
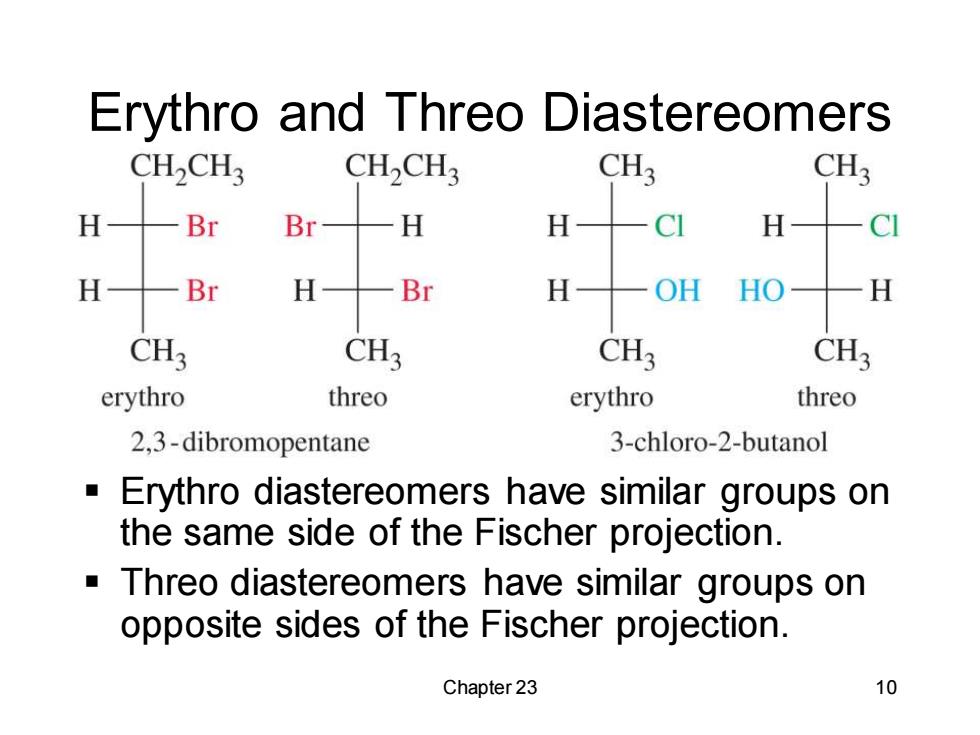
Erythro and Threo Diastereomers CH2CH3 CH2CH CH3 CH3 H Br Br H H CI H H Br H —Br H OH HO H CH3 CH3 CH3 CH3 erythro threo erythro threo 2,3-dibromopentane 3-chloro-2-butanol ■ Erythro diastereomers have similar groups on the same side of the Fischer projection. Threo diastereomers have similar groups on opposite sides of the Fischer projection. Chapter 23 10
Chapter 23 10 Erythro and Threo Diastereomers ▪ Erythro diastereomers have similar groups on the same side of the Fischer projection. ▪ Threo diastereomers have similar groups on opposite sides of the Fischer projection