
CNGNGE JOHN MCMURRY CHAPTER 5 Stereochemistry at Tetrahedral Centers T H I R D E DI TION Organic Chemistry with Biological Applications
CHAPTER 5 Stereochemistry at Tetrahedral Centers

Handedness Right and left hands are not identical Right and left hands are mirror images of each other they are nonsuperimposable mirror images Almost all the molecules in the human body are handed Handedness primarily arises from the tetrahedral stereochemistry of sp3-hybridized carbon atoms Handedness is crucial to understanding biological chemistry Left hand Right hand
Right and left hands are not identical ▪ Right and left hands are mirror images of each other – they are nonsuperimposable mirror images ▪ Almost all the molecules in the human body are handed ▪ Handedness primarily arises from the tetrahedral stereochemistry of sp3 -hybridized carbon atoms ▪ Handedness is crucial to understanding biological chemistry Handedness
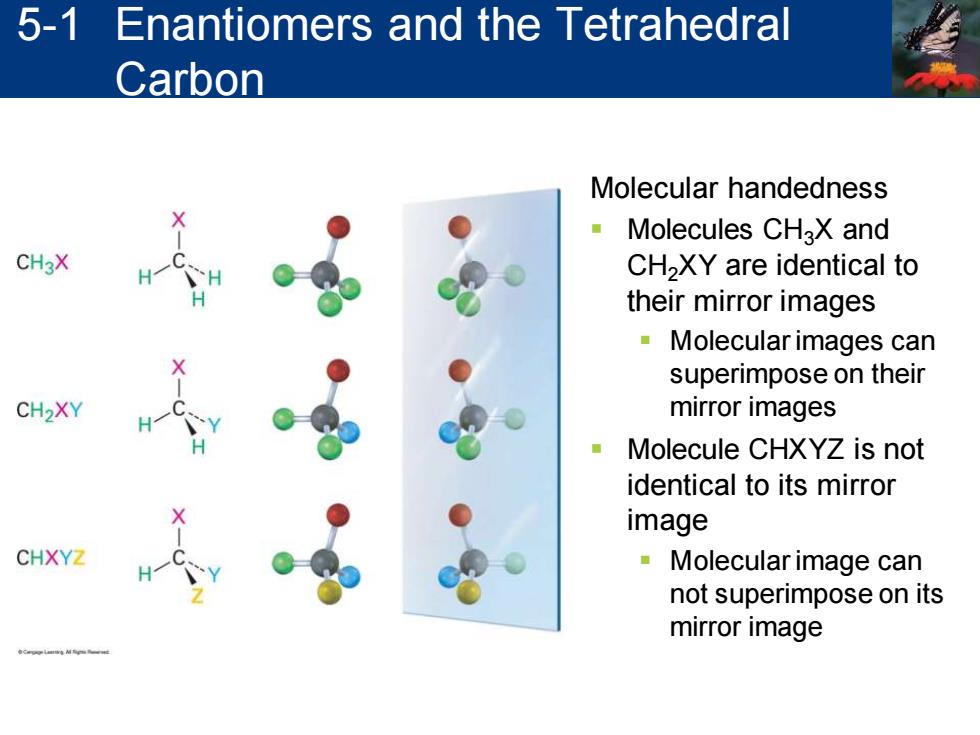
5-1 Enantiomers and the Tetrahedral Carbon Molecular handedness Molecules CHaX and CH3X CH2XY are identical to their mirror images 。Molecular images can superimpose on their CH2XY mirror images Molecule CHXYZ is not identical to its mirror image CHXYZ 。Molecular image can not superimpose on its mirror image
Molecular handedness ▪ Molecules CH3X and CH2XY are identical to their mirror images ▪ Molecular images can superimpose on their mirror images ▪ Molecule CHXYZ is not identical to its mirror image ▪ Molecular image can not superimpose on its mirror image 5-1 Enantiomers and the Tetrahedral Carbon
Enantiomers and the Tetrahedral Carbon Enantiomers "From the Greek enantio,meaning“opposite'” Stereoisomers in which molecules are not identical to their mirror images Result whenever a tetrahedral carbon is bonded to four different substituents CHXYZ (one need not be H) Lactic acid(2-hydroxypropanoic acid)has four different groups (-H,-OH,-CH3,-CO2H)bonded to the central carbon atoms and exists as a pair of enantiomers
Enantiomers ▪ From the Greek enantio, meaning “opposite” ▪ Stereoisomers in which molecules are not identical to their mirror images ▪ Result whenever a tetrahedral carbon is bonded to four different substituents CHXYZ (one need not be H) ▪ Lactic acid (2-hydroxypropanoic acid) has four different groups (-H, -OH, -CH3 , -CO2H) bonded to the central carbon atoms and exists as a pair of enantiomers Enantiomers and the Tetrahedral Carbon
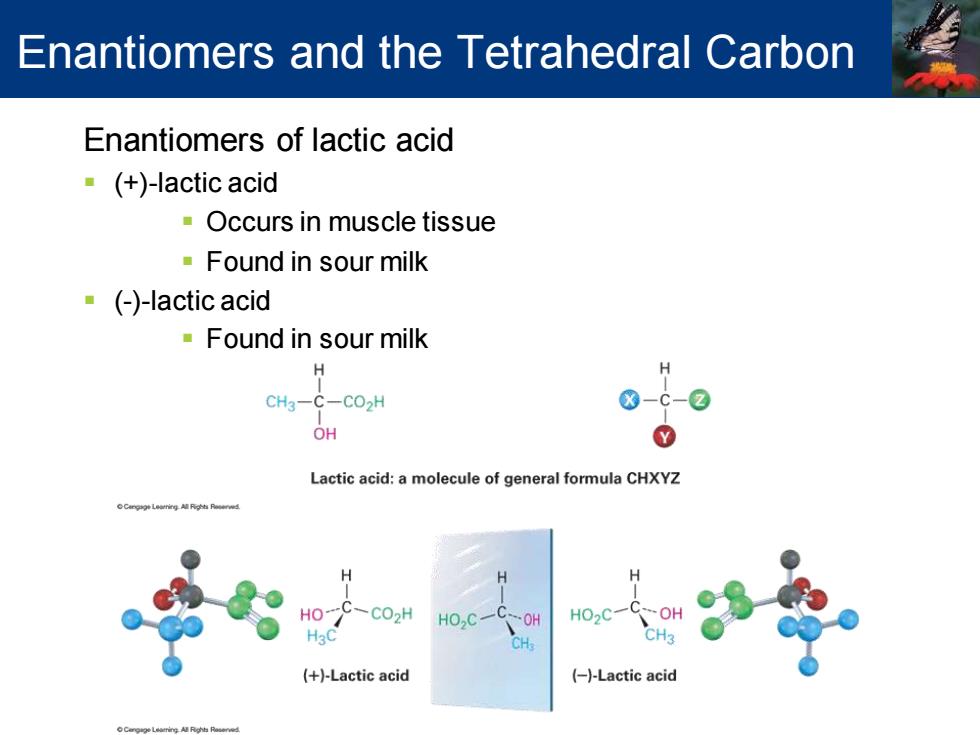
Enantiomers and the Tetrahedral Carbon Enantiomers of lactic acid (+)-lactic acid Occurs in muscle tissue Found in sour milk ()-lactic acid ·Found in sour milk H CH2- C-CO2H OH Lactic acid:a molecule of general formula CHXYZ H C02H HO,C- HO2C- (+)-Lactic acid (-)-Lactic acid
Enantiomers of lactic acid ▪ (+)-lactic acid ▪ Occurs in muscle tissue ▪ Found in sour milk ▪ (-)-lactic acid ▪ Found in sour milk Enantiomers and the Tetrahedral Carbon
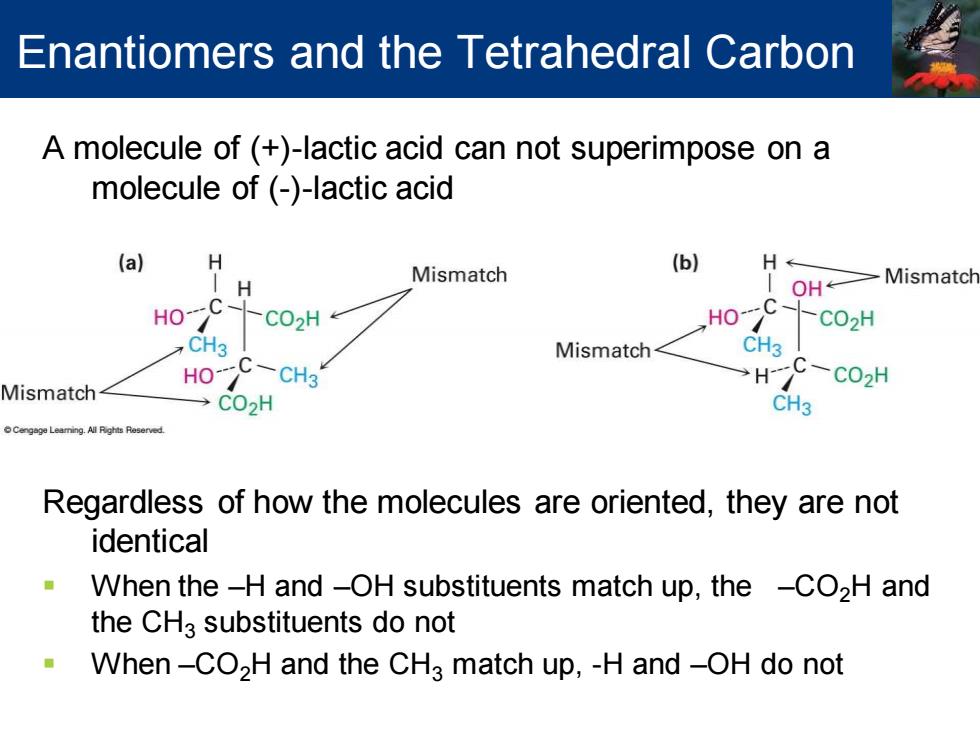
Enantiomers and the Tetrahedral Carbon A molecule of (+)-lactic acid can not superimpose on a molecule of (-)-lactic acid (a) Mismatch (b) H H OH Mismatch HO >C02H HO C -CO2H CH3 Mismatch C Mismatch HO-C-CH3 HC-CO2H →C02H CH3 Regardless of how the molecules are oriented,they are not identical When the-H and -OH substituents match up,the -CO2H and the CHa substituents do not When-CO2H and the CH3 match up,-H and-OH do not
A molecule of (+)-lactic acid can not superimpose on a molecule of (-)-lactic acid Regardless of how the molecules are oriented, they are not identical ▪ When the –H and –OH substituents match up, the –CO2H and the CH3 substituents do not ▪ When –CO2H and the CH3 match up, -H and –OH do not Enantiomers and the Tetrahedral Carbon
5-2 The Reason for Handedness in Molecules: Chirality Chiral -From the Greek cheir meaning "hand" Molecules that are not identical to their mirror images,and thus exist in two enantiomeric forms A molecule is not chiral if it has a plane of symmetry Plane of symmetry A plane that cuts through the middle of an object (or molecule)so that one half of the object is a mirror image of the other half
Chiral ▪ From the Greek cheir meaning “hand” ▪ Molecules that are not identical to their mirror images, and thus exist in two enantiomeric forms ▪ A molecule is not chiral if it has a plane of symmetry Plane of symmetry ▪ A plane that cuts through the middle of an object (or molecule) so that one half of the object is a mirror image of the other half 5-2 The Reason for Handedness in Molecules: Chirality
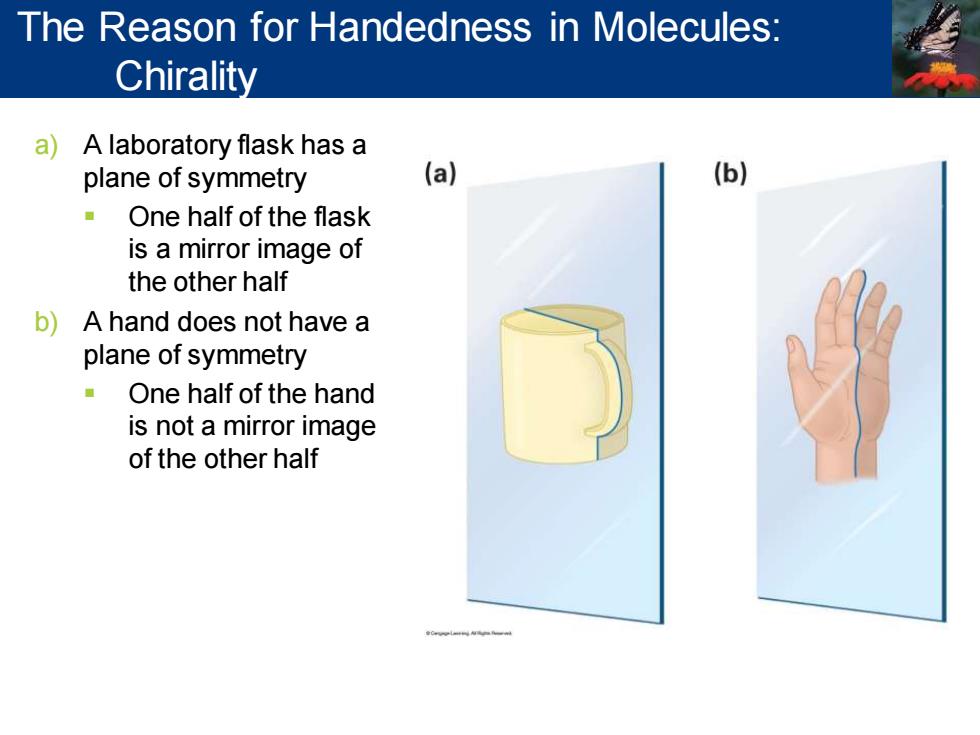
The Reason for Handedness in Molecules: Chirality a)A laboratory flask has a plane of symmetry (a) (b) One half of the flask is a mirror image of the other half b)A hand does not have a plane of symmetry One half of the hand is not a mirror image of the other half FORWLEINNAPm
a) A laboratory flask has a plane of symmetry ▪ One half of the flask is a mirror image of the other half b) A hand does not have a plane of symmetry ▪ One half of the hand is not a mirror image of the other half The Reason for Handedness in Molecules: Chirality
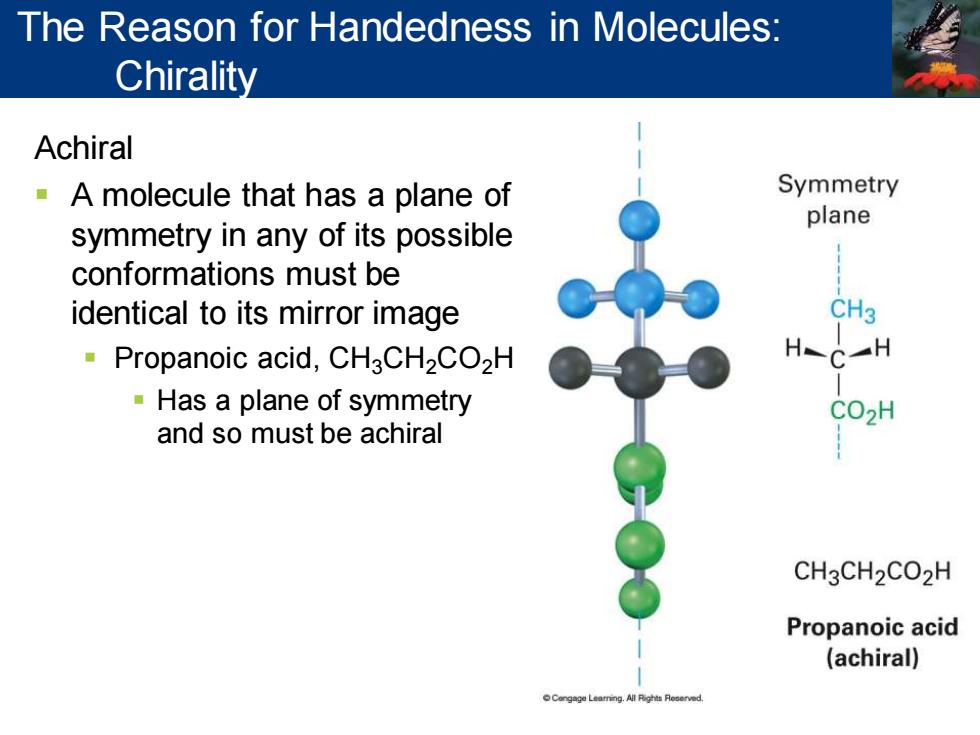
The Reason for Handedness in Molecules: Chirality Achiral -A molecule that has a plane of Symmetry plane symmetry in any of its possible conformations must be identical to its mirror image CH3 Propanoic acid,CH3CH2CO2H HC一H Has a plane of symmetry CO2H and so must be achiral CH3CH2CO2H Propanoic acid (achiral) Cengage Leaming.All Fighis Reoerved
Achiral ▪ A molecule that has a plane of symmetry in any of its possible conformations must be identical to its mirror image ▪ Propanoic acid, CH3CH2CO2H ▪ Has a plane of symmetry and so must be achiral The Reason for Handedness in Molecules: Chirality
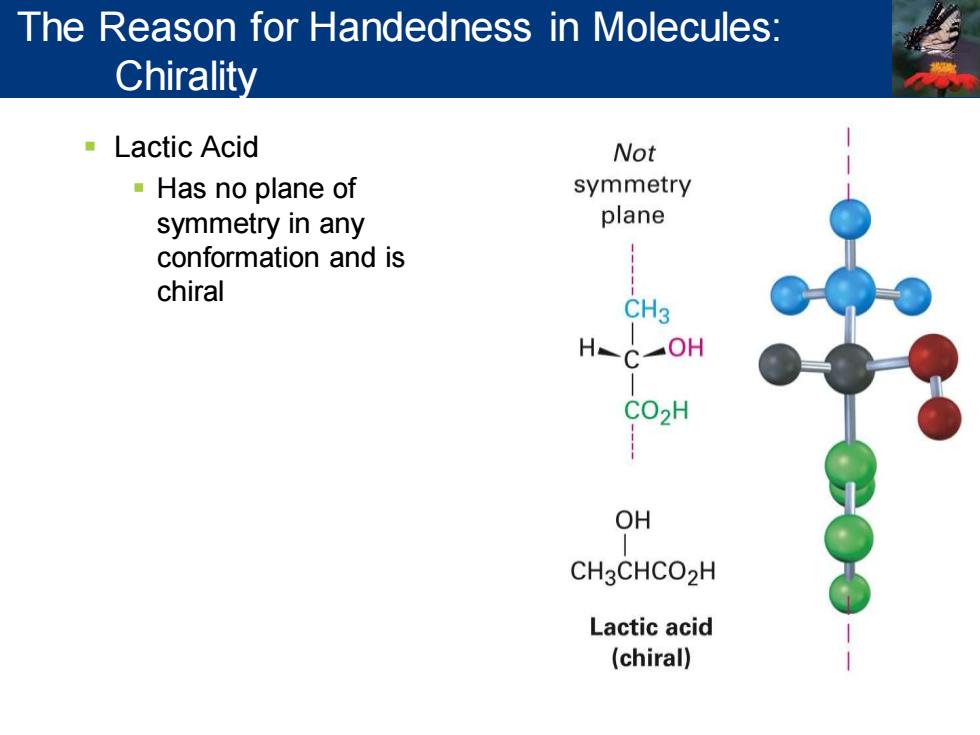
The Reason for Handedness in Molecules: Chirality ■Lactic Acid Not Has no plane of symmetry symmetry in any plane conformation and is chiral CH3 OH CO2H OH CH3CHCO2H Lactic acid (chiral)
▪ Lactic Acid ▪ Has no plane of symmetry in any conformation and is chiral The Reason for Handedness in Molecules: Chirality