
CNGNGE JOHN MCMURRY CHAPTER 18 Amines and Heterocycles T H I R D E DIT ION Organic Chemistry with Biological Applications
CHAPTER 18 Amines and Heterocycles
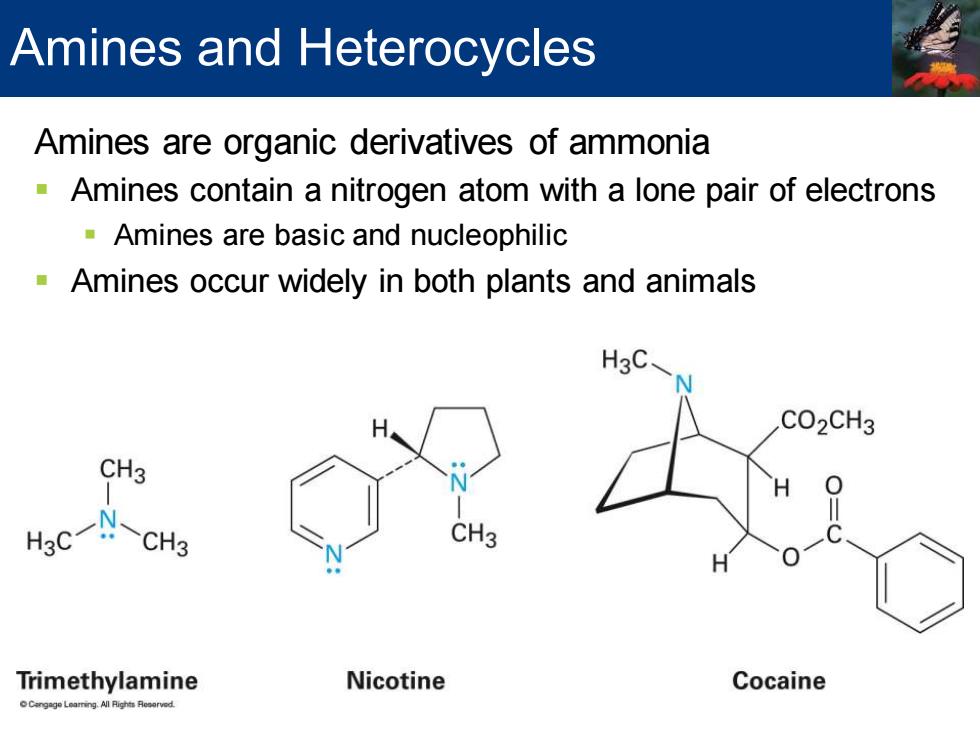
Amines and Heterocycles Amines are organic derivatives of ammonia Amines contain a nitrogen atom with a lone pair of electrons Amines are basic and nucleophilic Amines occur widely in both plants and animals H3C CO2CH3 CH3 CH3 Trimethylamine Nicotine Cocaine angage Learring Al Rights Resorved
Amines are organic derivatives of ammonia ▪ Amines contain a nitrogen atom with a lone pair of electrons ▪ Amines are basic and nucleophilic ▪ Amines occur widely in both plants and animals Amines and Heterocycles
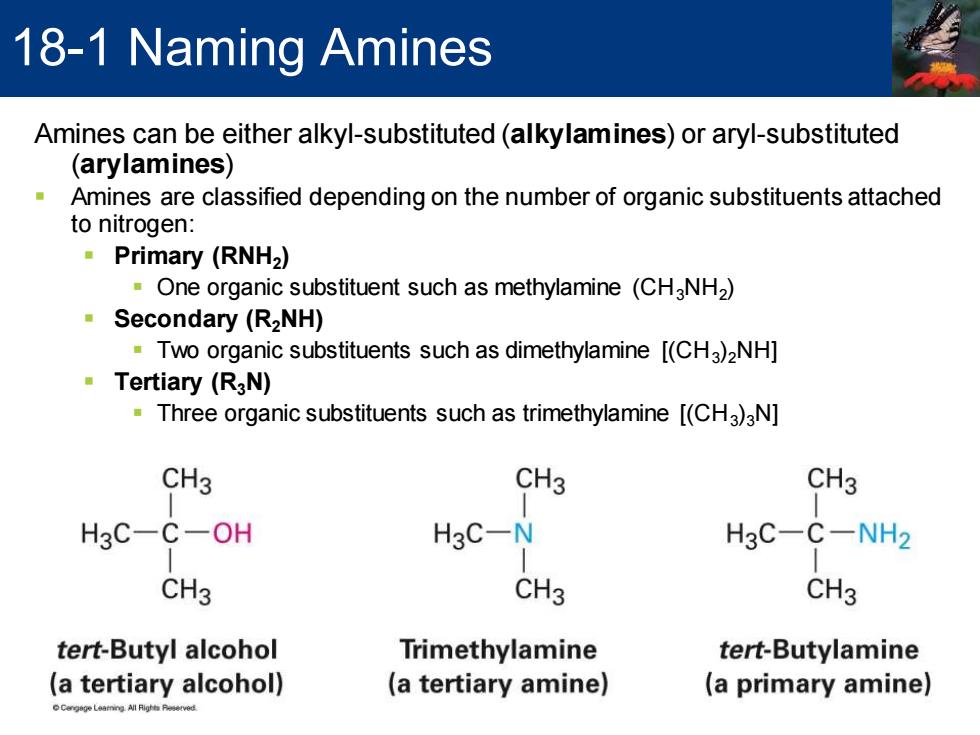
18-1 Naming Amines Amines can be either alkyl-substituted(alkylamines)or aryl-substituted (arylamines) Amines are classified depending on the number of organic substituents attached to nitrogen: ·Primary(RNH2) One organic substituent such as methylamine (CH3NH2) ·Secondary(RzNH) Two organic substituents such as dimethylamine [(CHa)2NH] 。Tertiary(RgN) Three organic substituents such as trimethylamine [(CHa)aN] CH3 CH3 CH3 H3C-C-OH H3C-N H3C-C-NH2 CH3 CH3 CH3 tert-Butyl alcohol Trimethylamine tert-Butylamine (a tertiary alcohol) (a tertiary amine) (a primary amine)
Amines can be either alkyl-substituted (alkylamines) or aryl-substituted (arylamines) ▪ Amines are classified depending on the number of organic substituents attached to nitrogen: ▪ Primary (RNH2 ) ▪ One organic substituent such as methylamine (CH3NH2 ) ▪ Secondary (R2NH) ▪ Two organic substituents such as dimethylamine [(CH3 )2NH] ▪ Tertiary (R3N) ▪ Three organic substituents such as trimethylamine [(CH3 )3N] 18-1 Naming Amines
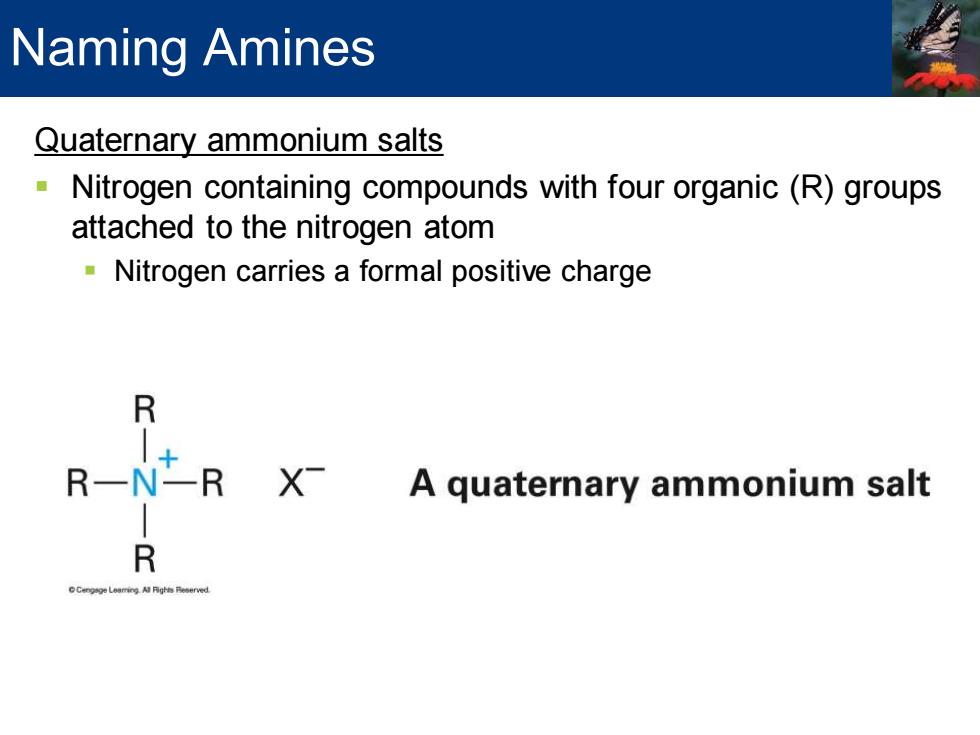
Naming Amines Quaternary ammonium salts Nitrogen containing compounds with four organic(R)groups attached to the nitrogen atom Nitrogen carries a formal positive charge R R一 -R X A quaternary ammonium salt R
Quaternary ammonium salts ▪ Nitrogen containing compounds with four organic (R) groups attached to the nitrogen atom ▪ Nitrogen carries a formal positive charge Naming Amines
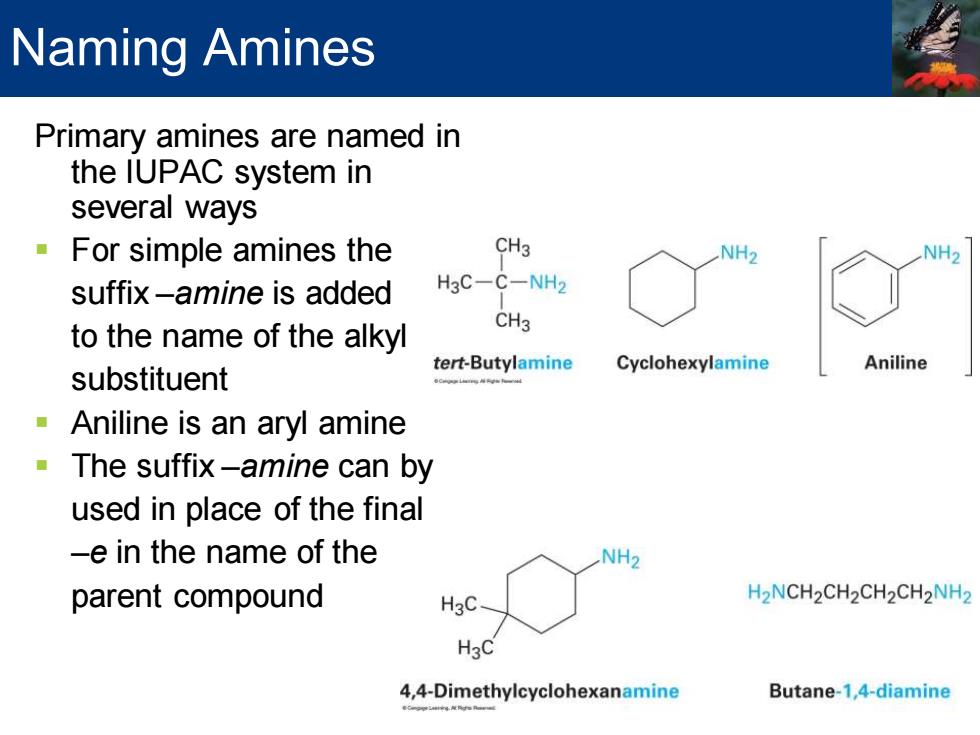
Naming Amines Primary amines are named in the IUPAC system in several ways For simple amines the CH3 suffix-amine is added H3C-C-NH2 to the name of the alkyl CH3 tert-Butylamine Cyclohexylamine Aniline substituent Aniline is an aryl amine The suffix-amine can by used in place of the final -e in the name of the NH2 parent compound H2NCH2CH2CH2CH2NH2 H3C 4,4-Dimethylcyclohexanamine Butane-1,4-diamine
Primary amines are named in the IUPAC system in several ways ▪ For simple amines the suffix –amine is added to the name of the alkyl substituent ▪ Aniline is an aryl amine ▪ The suffix –amine can by used in place of the final –e in the name of the parent compound Naming Amines
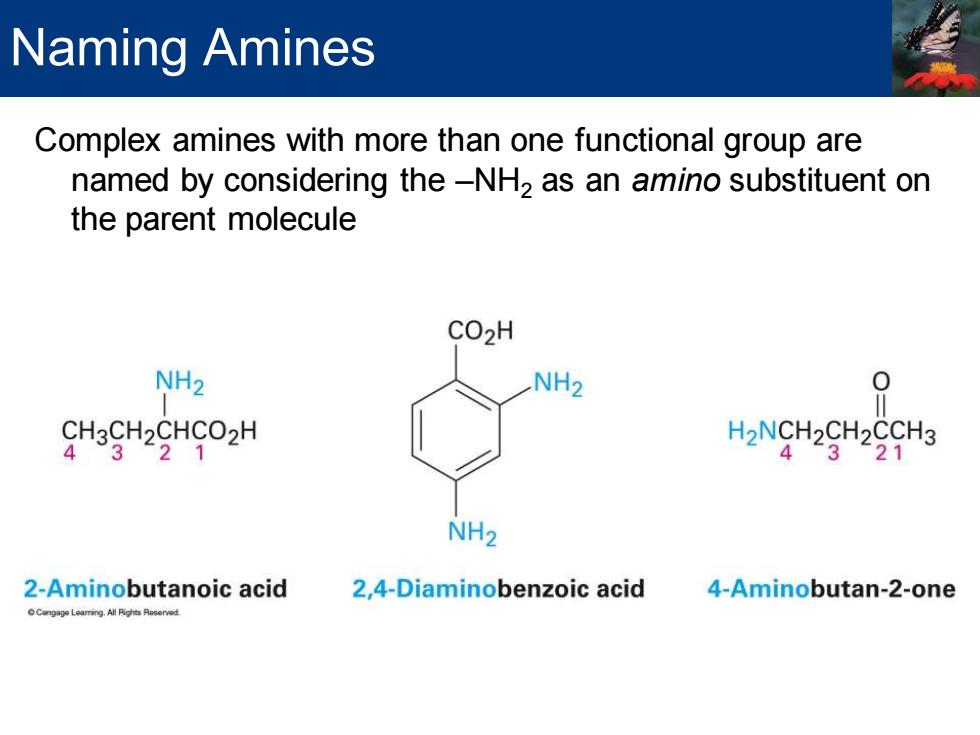
Naming Amines Complex amines with more than one functional group are named by considering the-NH2 as an amino substituent on the parent molecule CO2H NH2 NH2 CH3CH2CHCO2H H2NCH2CH2CCH3 4321 4321 NH2 2-Aminobutanoic acid 2,4-Diaminobenzoic acid 4-Aminobutan-2-one
Complex amines with more than one functional group are named by considering the –NH2 as an amino substituent on the parent molecule Naming Amines
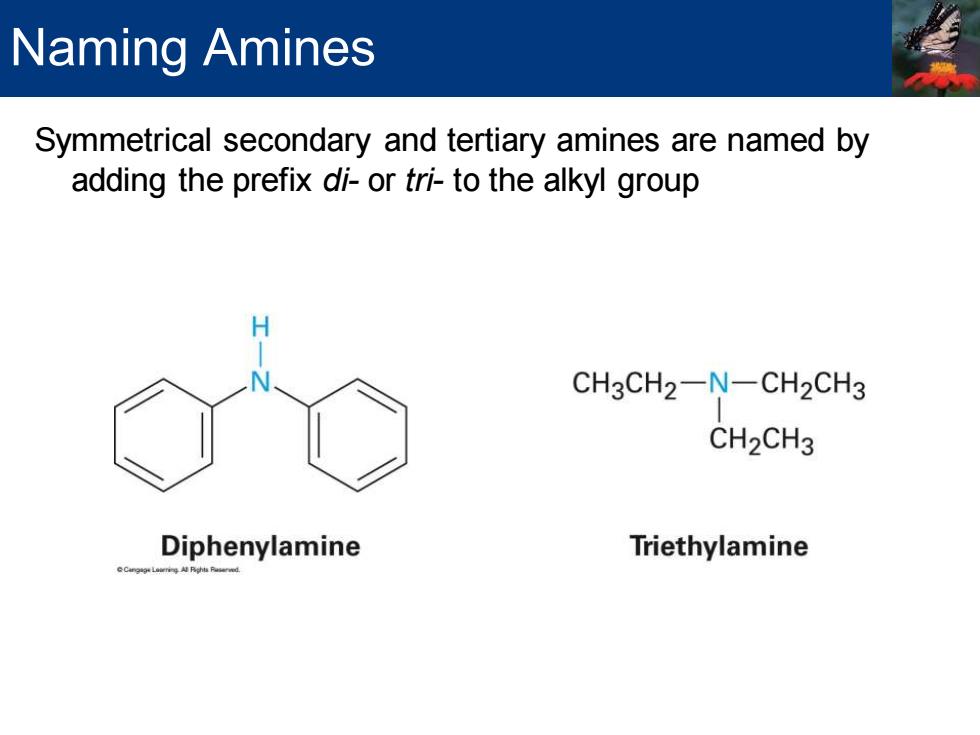
Naming Amines Symmetrical secondary and tertiary amines are named by adding the prefix di-or tri-to the alkyl group CH3CH2-N-CH2CH3 CH2CH3 Diphenylamine Triethylamine
Symmetrical secondary and tertiary amines are named by adding the prefix di- or tri- to the alkyl group Naming Amines
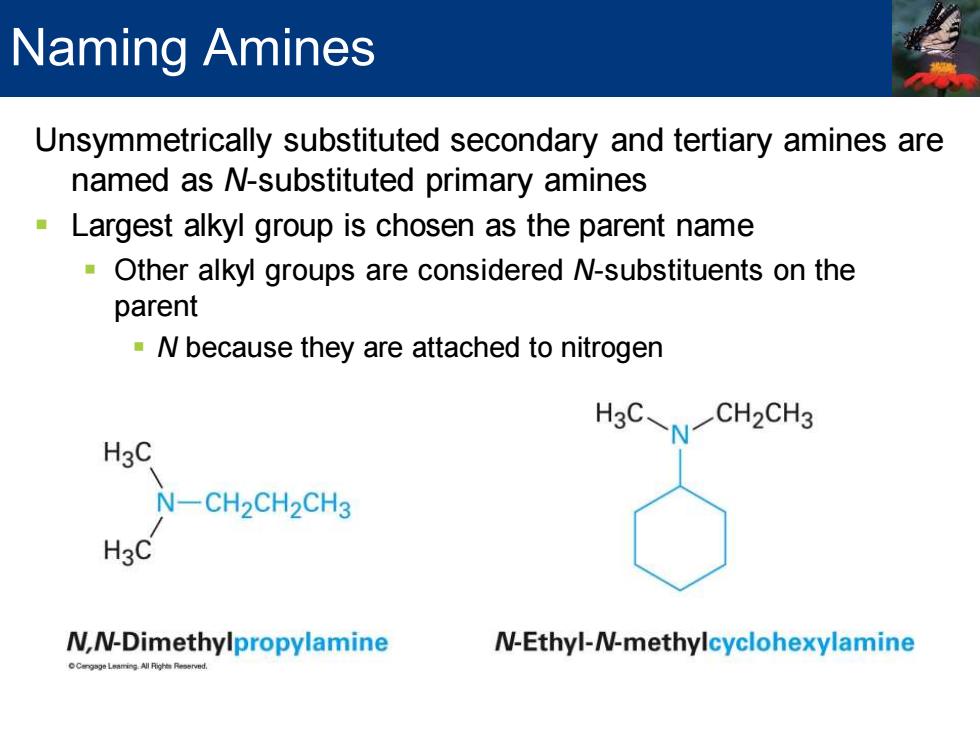
Naming Amines Unsymmetrically substituted secondary and tertiary amines are named as N-substituted primary amines Largest alkyl group is chosen as the parent name Other alkyl groups are considered N-substituents on the parent N because they are attached to nitrogen CH2CH3 H3C N-CH2CH2CH3 HgC N,N-Dimethylpropylamine N-Ethyl-N-methylcyclohexylamine
Unsymmetrically substituted secondary and tertiary amines are named as N-substituted primary amines ▪ Largest alkyl group is chosen as the parent name ▪ Other alkyl groups are considered N-substituents on the parent ▪ N because they are attached to nitrogen Naming Amines
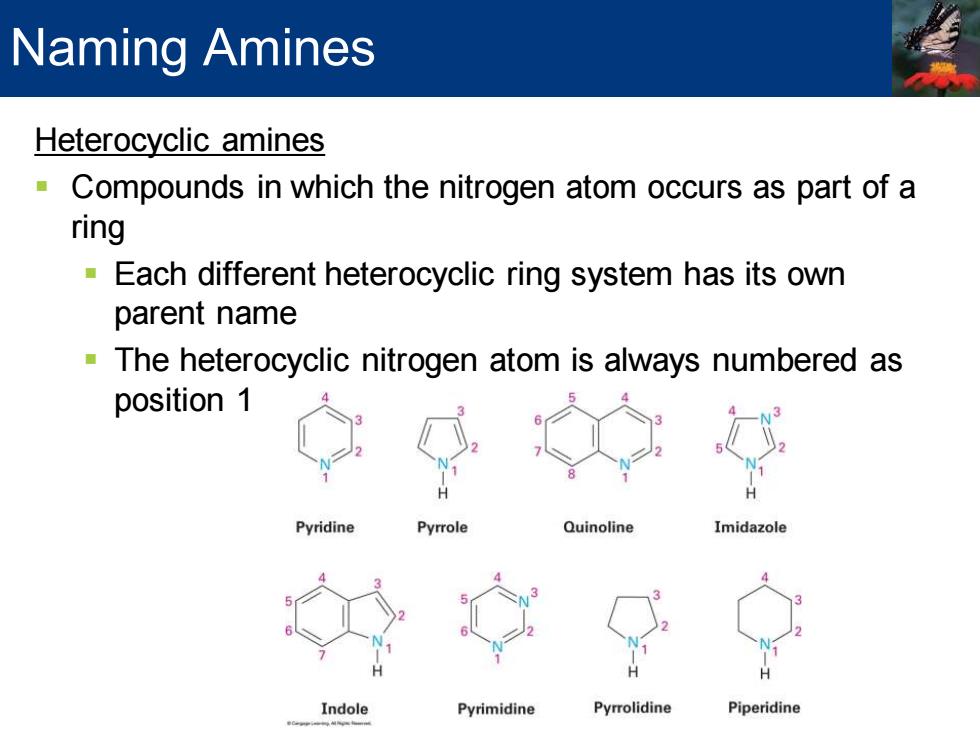
Naming Amines Heterocyclic amines Compounds in which the nitrogen atom occurs as part of a ring Each different heterocyclic ring system has its own parent name -The heterocyclic nitrogen atom is always numbered as position 1 Pyridine Pyrrole Quinoline Imidazole Indole Pyrimidine Pyrrolidine Piperidine
Heterocyclic amines ▪ Compounds in which the nitrogen atom occurs as part of a ring ▪ Each different heterocyclic ring system has its own parent name ▪ The heterocyclic nitrogen atom is always numbered as position 1 Naming Amines
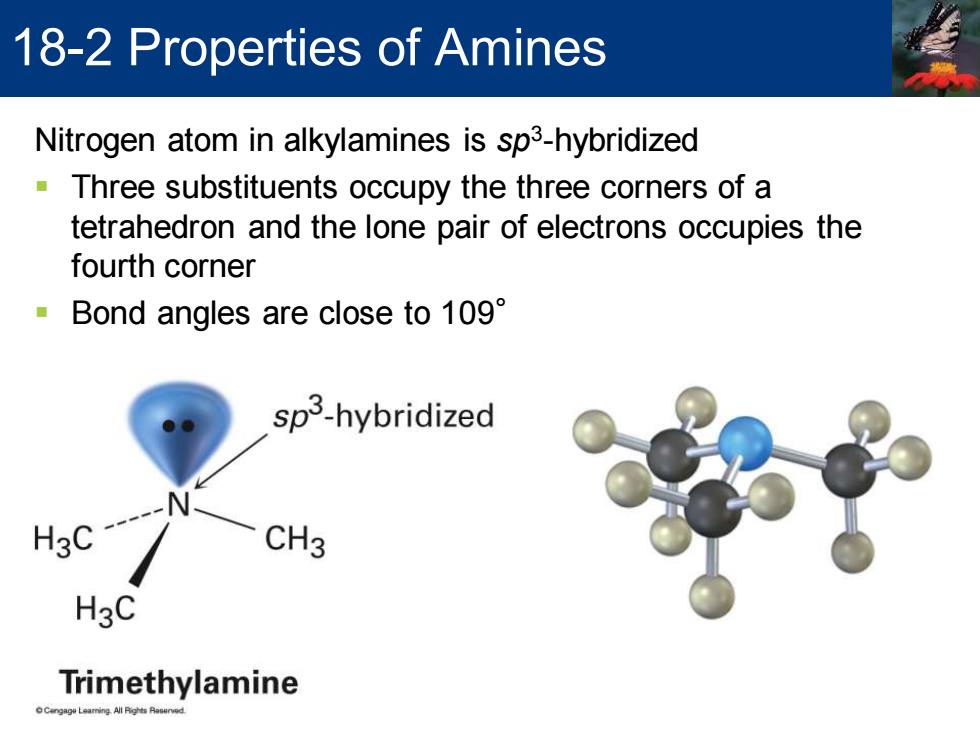
18-2 Properties of Amines Nitrogen atom in alkylamines is sp3-hybridized Three substituents occupy the three corners of a tetrahedron and the lone pair of electrons occupies the fourth corner Bond angles are close to 109 sp3-hybridized HgC CH3 H3C Trimethylamine
Nitrogen atom in alkylamines is sp3 -hybridized ▪ Three substituents occupy the three corners of a tetrahedron and the lone pair of electrons occupies the fourth corner ▪ Bond angles are close to 109° 18-2 Properties of Amines