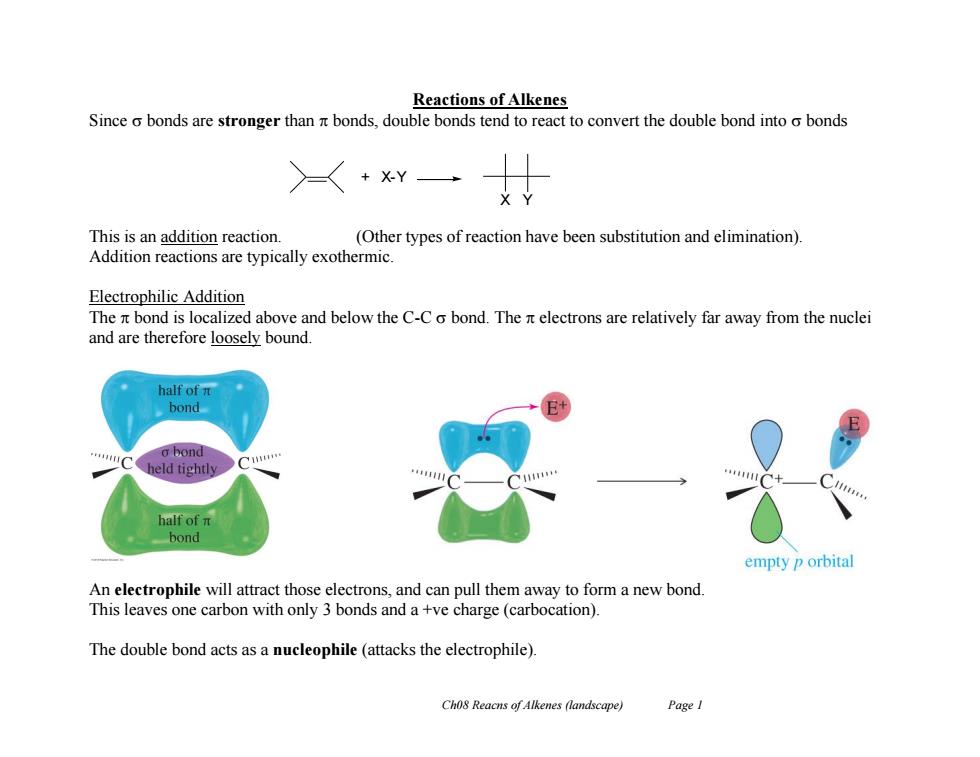
Reactions of Alkenes Since o bonds are stronger than nt bonds,double bonds tend to react to convert the double bond into o bonds >+xY This is an addition reaction (Other types of reaction have been substitution and elimination). Addition reactions are typically exothermic. Electrophilic Addition The it bond is localized above and below the C-C o bond.The it electrons are relatively far away from the nuclei and are therefore loosely bound. half of n bond o bond held tightly half of n bond empty p orbital An electrophile will attract those electrons,and can pull them away to form a new bond. This leaves one carbon with only 3 bonds and a +ve charge (carbocation). The double bond acts as a nucleophile(attacks the electrophile). Cho8 Reacns of Alkenes (landscape) Page I
Ch08 Reacns of Alkenes (landscape) Page 1 Reactions of Alkenes Since bonds are stronger than bonds, double bonds tend to react to convert the double bond into bonds This is an addition reaction. (Other types of reaction have been substitution and elimination). Addition reactions are typically exothermic. Electrophilic Addition The bond is localized above and below the C-C bond. The electrons are relatively far away from the nuclei and are therefore loosely bound. An electrophile will attract those electrons, and can pull them away to form a new bond. This leaves one carbon with only 3 bonds and a +ve charge (carbocation). The double bond acts as a nucleophile (attacks the electrophile). + X-Y X Y
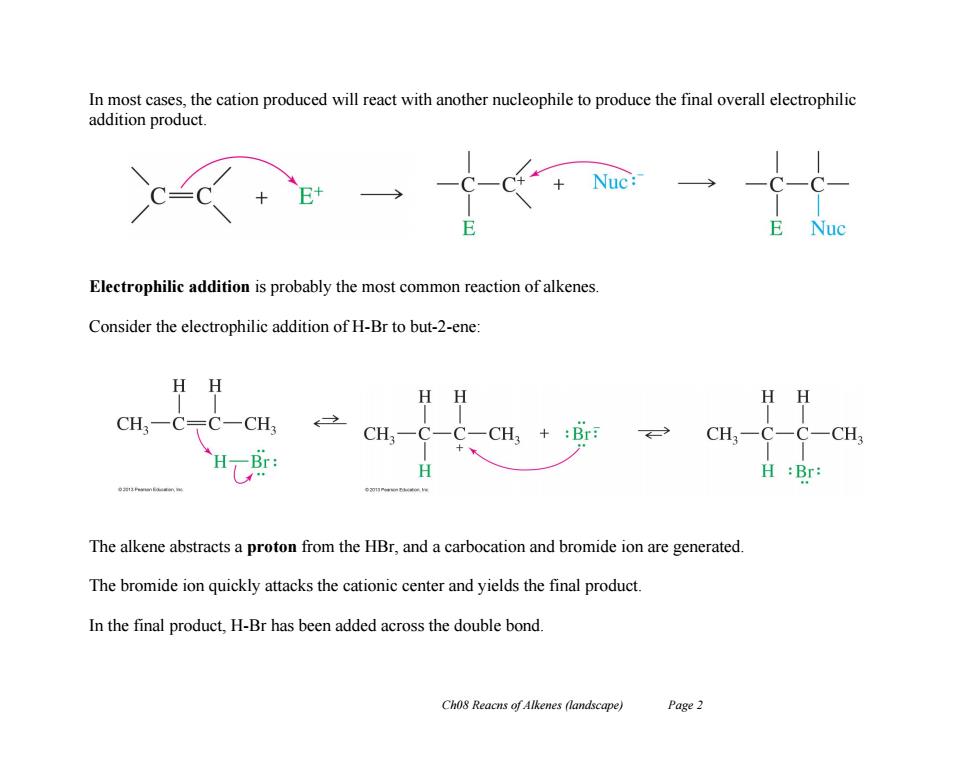
In most cases,the cation produced will react with another nucleophile to produce the final overall electrophilic addition product. Electrophilic addition is probably the most common reaction of alkenes. Consider the electrophilic addition of H-Br to but-2-ene: H HH CH. CH. CH, +:Br CH3一C-C-CH3 Br H:Br: The alkene abstracts a proton from the HBr,and a carbocation and bromide ion are generated. The bromide ion quickly attacks the cationic center and yields the final product. In the final product,H-Br has been added across the double bond. Cho8 Reacns of Alkenes (landscape) Page 2
Ch08 Reacns of Alkenes (landscape) Page 2 In most cases, the cation produced will react with another nucleophile to produce the final overall electrophilic addition product. Electrophilic addition is probably the most common reaction of alkenes. Consider the electrophilic addition of H-Br to but-2-ene: The alkene abstracts a proton from the HBr, and a carbocation and bromide ion are generated. The bromide ion quickly attacks the cationic center and yields the final product. In the final product, H-Br has been added across the double bond
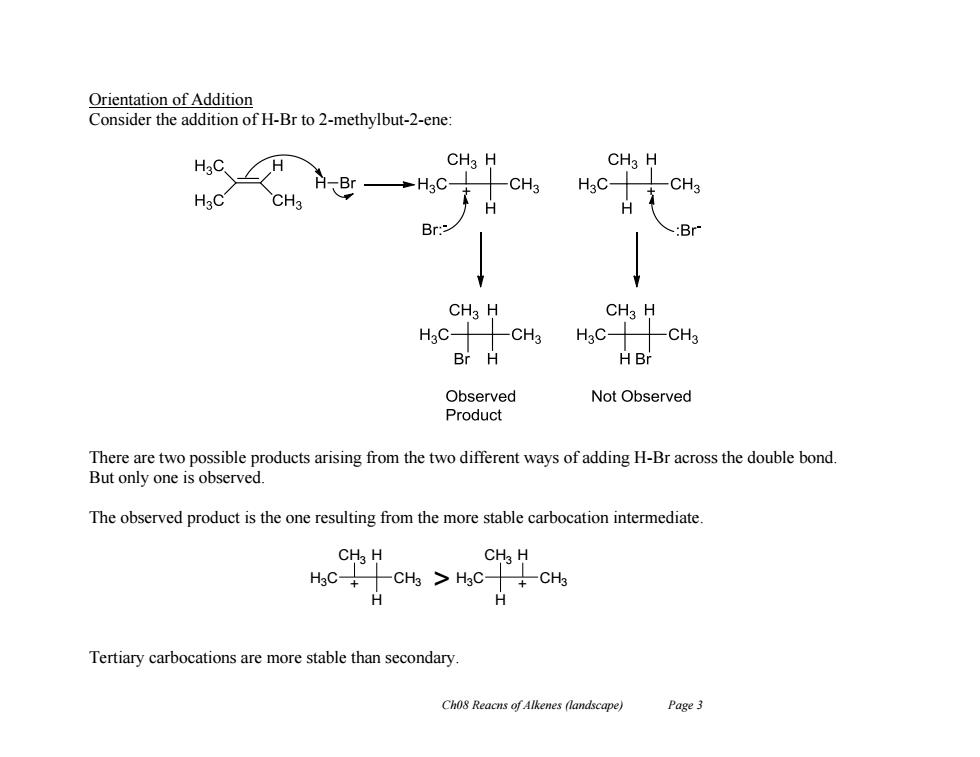
Orientation of Addition Consider the addition of H-Br to 2-methylbut-2-ene: CHa H CHa H -CH3 H3C- -CH3 CH3 HBr→HC H Br: :Br CH3 H CH3 H H3C- CH3 HgC-CH3 Br H HBr Observed Not Observed Product There are two possible products arising from the two different ways of adding H-Br across the double bond. But only one is observed. The observed product is the one resulting from the more stable carbocation intermediate. CH H CH H HaCCHs HgC-+CH3 H H Tertiary carbocations are more stable than secondary. Cho8 Reacns of Alkenes (landscape) Page 3
Ch08 Reacns of Alkenes (landscape) Page 3 Orientation of Addition Consider the addition of H-Br to 2-methylbut-2-ene: There are two possible products arising from the two different ways of adding H-Br across the double bond. But only one is observed. The observed product is the one resulting from the more stable carbocation intermediate. Tertiary carbocations are more stable than secondary. H3C CH3 H CH3 H + CH3 CH3 H H3C H > +
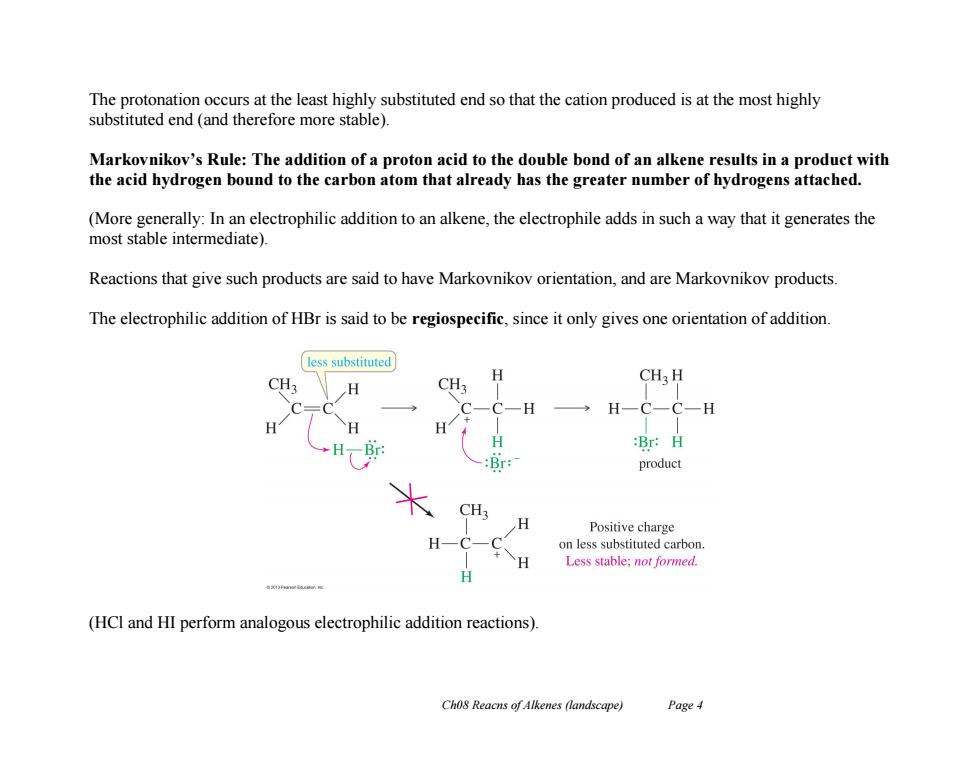
The protonation occurs at the least highly substituted end so that the cation produced is at the most highly substituted end (and therefore more stable). Markovnikov's Rule:The addition of a proton acid to the double bond of an alkene results in a product with the acid hydrogen bound to the carbon atom that already has the greater number of hydrogens attached. (More generally:In an electrophilic addition to an alkene,the electrophile adds in such a way that it generates the most stable intermediate). Reactions that give such products are said to have Markovnikov orientation,and are Markovnikov products. The electrophilic addition of HBr is said to be regiospecific,since it only gives one orientation of addition. less substituted CH3 H H CH H -Br :Br:H :Br: product CH H Positive charge H C-H on less substituted carbon. Less stable;not formed. H (HCI and HI perform analogous electrophilic addition reactions). Cho8 Reacns of Alkenes (landscape) Page 4
Ch08 Reacns of Alkenes (landscape) Page 4 The protonation occurs at the least highly substituted end so that the cation produced is at the most highly substituted end (and therefore more stable). Markovnikov’s Rule: The addition of a proton acid to the double bond of an alkene results in a product with the acid hydrogen bound to the carbon atom that already has the greater number of hydrogens attached. (More generally: In an electrophilic addition to an alkene, the electrophile adds in such a way that it generates the most stable intermediate). Reactions that give such products are said to have Markovnikov orientation, and are Markovnikov products. The electrophilic addition of HBr is said to be regiospecific, since it only gives one orientation of addition. (HCl and HI perform analogous electrophilic addition reactions)

Free Radical addition to Alkenes It is possible to obtain anti-Markovnikov products when HBr is added to alkenes in the presence of free radical initiators. The free radical initiators change the mechanism of addition from electrophilic addition to free radical addition. This change of mechanism gives rise to the opposite regiochemistry Initiation: 6-R R-+·-R R-0+H- R-O一H+:Br The oxygen-oxygen bond is weak,and is easily homolytically cleaved to generate two alkoxy radicals,which in turn abstract hydrogen to generate bromine radicals. Cho8 Reacns of Alkenes (landscape) Page 5
Ch08 Reacns of Alkenes (landscape) Page 5 Free Radical addition to Alkenes It is possible to obtain anti-Markovnikov products when HBr is added to alkenes in the presence of free radical initiators. The free radical initiators change the mechanism of addition from electrophilic addition to free radical addition. This change of mechanism gives rise to the opposite regiochemistry. Initiation: The oxygen-oxygen bond is weak, and is easily homolytically cleaved to generate two alkoxy radicals, which in turn abstract hydrogen to generate bromine radicals
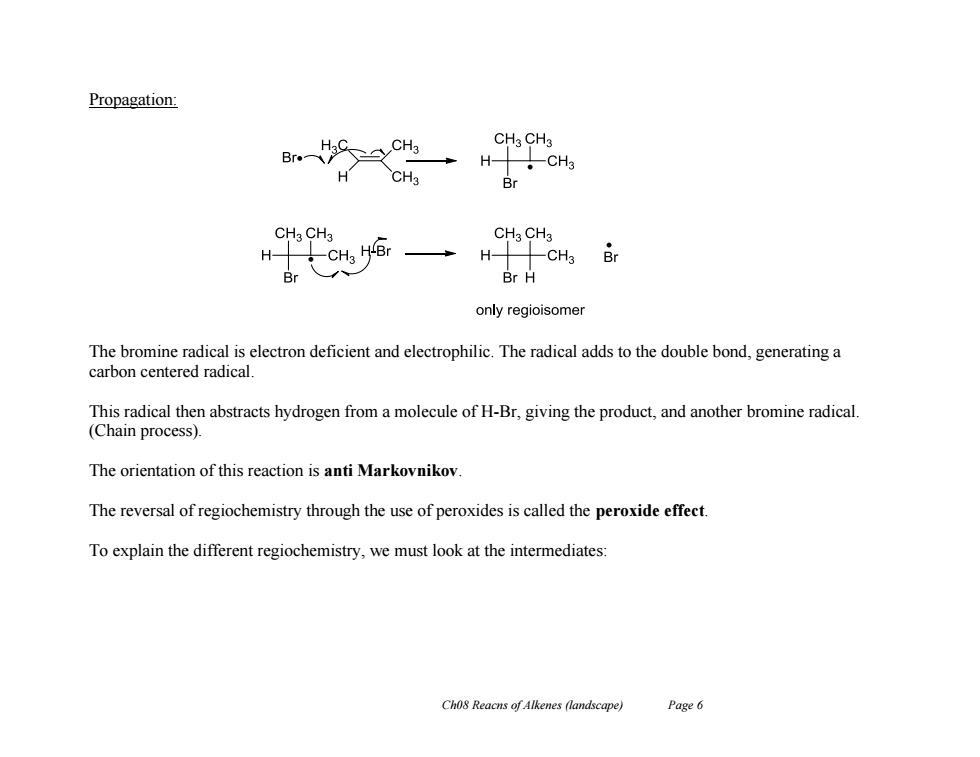
Propagation: CHa CH3 Br H CH3 H-11CH Br CHa CH3 CHaCH3 H十CH 5 Br Br H only regioisomer The bromine radical is electron deficient and electrophilic.The radical adds to the double bond,generating a carbon centered radical. This radical then abstracts hydrogen from a molecule of H-Br,giving the product,and another bromine radical. (Chain process). The orientation of this reaction is anti Markovnikov The reversal of regiochemistry through the use of peroxides is called the peroxide effect. To explain the different regiochemistry,we must look at the intermediates: Ch08 Reacns of Alkenes (landscape) Page 6
Ch08 Reacns of Alkenes (landscape) Page 6 Propagation: The bromine radical is electron deficient and electrophilic. The radical adds to the double bond, generating a carbon centered radical. This radical then abstracts hydrogen from a molecule of H-Br, giving the product, and another bromine radical. (Chain process). The orientation of this reaction is anti Markovnikov. The reversal of regiochemistry through the use of peroxides is called the peroxide effect. To explain the different regiochemistry, we must look at the intermediates:
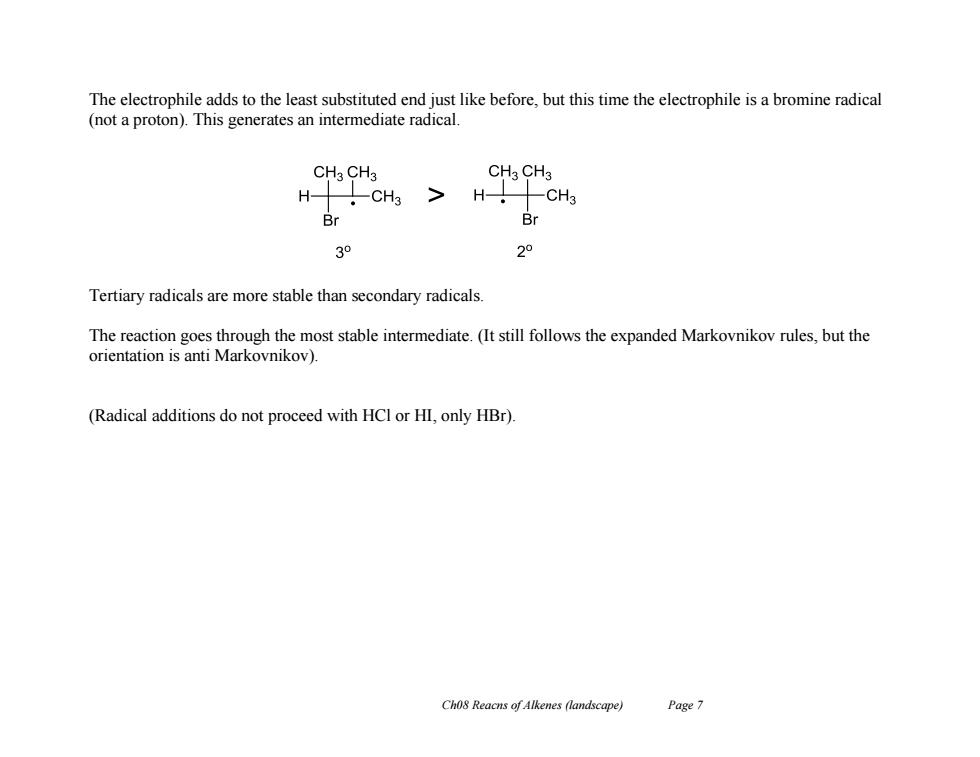
The electrophile adds to the least substituted end just like before,but this time the electrophile is a bromine radical (not a proton).This generates an intermediate radical. CH3 CH3 CH3 CH3 H H- CH3 Br 03o 2° Tertiary radicals are more stable than secondary radicals. The reaction goes through the most stable intermediate.(It still follows the expanded Markovnikov rules,but the orientation is anti Markovnikov). (Radical additions do not proceed with HCl or HI,only HBr). Ch08 Reacns of Alkenes (landscape) Page 7
Ch08 Reacns of Alkenes (landscape) Page 7 The electrophile adds to the least substituted end just like before, but this time the electrophile is a bromine radical (not a proton). This generates an intermediate radical. Tertiary radicals are more stable than secondary radicals. The reaction goes through the most stable intermediate. (It still follows the expanded Markovnikov rules, but the orientation is anti Markovnikov). (Radical additions do not proceed with HCl or HI, only HBr)

Addition of Water Alkenes can be converted to alcohols. It is the reverse reaction of the dehydration of alcohols to give alkenes +H20= H OH The principle of microscopic reversibility states that a forward reaction and a reverse reaction taking place under the same conditions must follow the same reaction pathway in microscopic detail (Logically,it seems sensible that the lowest energy T.S.'s and intermediates for the forward reaction would be the same for the reverse reaction but in the opposite order). So it is no surprise that the mechanism for hydration of alkenes is identical to that of dehydration of alcohols,but in the reverse order of steps. Ch08 Reacns of Alkenes (landscape) Page 8
Ch08 Reacns of Alkenes (landscape) Page 8 Addition of Water Alkenes can be converted to alcohols. It is the reverse reaction of the dehydration of alcohols to give alkenes. The principle of microscopic reversibility states that a forward reaction and a reverse reaction taking place under the same conditions must follow the same reaction pathway in microscopic detail. (Logically, it seems sensible that the lowest energy T.S.’s and intermediates for the forward reaction would be the same for the reverse reaction but in the opposite order). So it is no surprise that the mechanism for hydration of alkenes is identical to that of dehydration of alcohols, but in the reverse order of steps. H OH + H2O
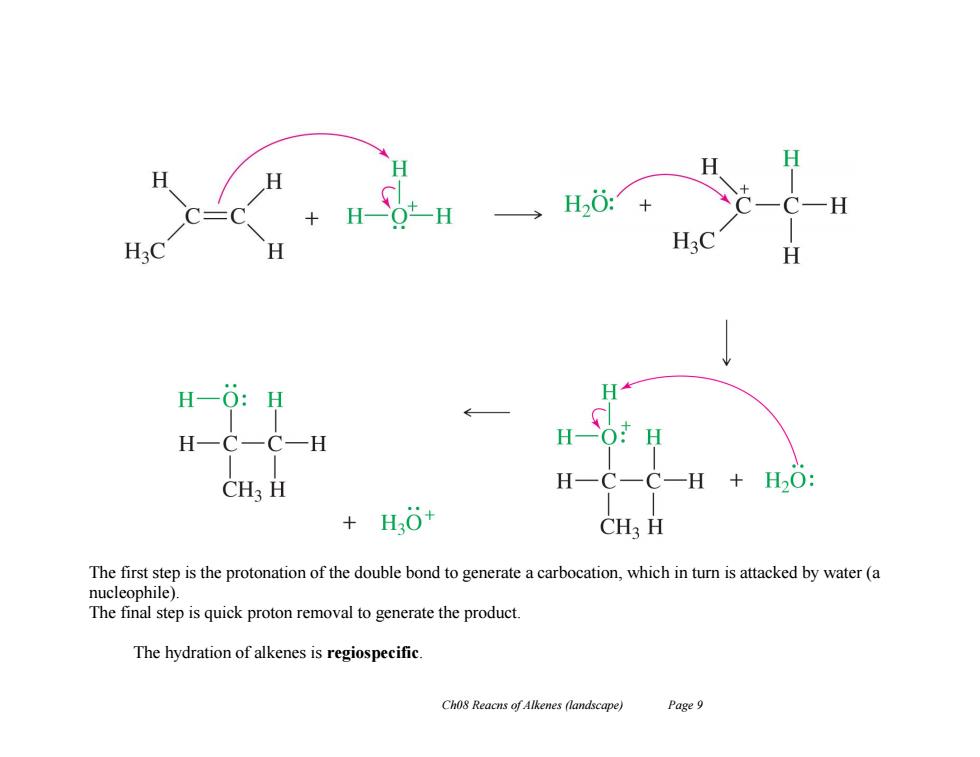
H一Ot-H H20:+ H20 H20 H H-O:H H H一C一C一H H一O:H CH3 H H-C-C一H+H2O: +H0+ CH3 H The first step is the protonation of the double bond to generate a carbocation,which in turn is attacked by water(a nucleophile). The final step is quick proton removal to generate the product. The hydration of alkenes is regiospecific. Ch08 Reacns of Alkenes (landscape) Page 9
Ch08 Reacns of Alkenes (landscape) Page 9 The first step is the protonation of the double bond to generate a carbocation, which in turn is attacked by water (a nucleophile). The final step is quick proton removal to generate the product. The hydration of alkenes is regiospecific

The orientation is Markovnikov since the proton has added to the least highly substituted end,and the hydroxyl to the most highly substituted end. The regiochemistry is explained by the intermediate carbocation: HH HH H + -CH3 H CH3 H 2° 10 The secondary carbocation is more stable than the primary carbocation The reaction of dilute acid to hydrate alkenes is not a fantastic practical route due to insolubility problems,and typically two other indirect approaches are used. (1)Addition of sulfuric acid followed by hydrolysis (2)Oxymercuration-demercuration Addition of Sulfuric Acid followed by Hydrolysis The alkene reacts with conc.sulfuric acid to give an alkyl hydrogen sulfate,which then in turn is hydrolyzed to give the alcohol. +H2SO4 H20 H OSO3H H OH alkene alkyl hydrogen alcohol sulfate Cho8 Reacns of Alkenes (landscape) Page 10
Ch08 Reacns of Alkenes (landscape) Page 10 The orientation is Markovnikov since the proton has added to the least highly substituted end, and the hydroxyl to the most highly substituted end. The regiochemistry is explained by the intermediate carbocation: The secondary carbocation is more stable than the primary carbocation. The reaction of dilute acid to hydrate alkenes is not a fantastic practical route due to insolubility problems, and typically two other indirect approaches are used. (1) Addition of sulfuric acid followed by hydrolysis (2) Oxymercuration-demercuration Addition of Sulfuric Acid followed by Hydrolysis The alkene reacts with conc. sulfuric acid to give an alkyl hydrogen sulfate, which then in turn is hydrolyzed to give the alcohol. H OSO3H + H2SO4 H OH H2O alkene alkyl hydrogen sulfate alcohol