
Organic Chemistry, ORGANIC 8th Edition CHEMISTRY L.G.Wade,Jr. Chapter 2 Lecture Structure and Properties of Organic Molecules .WAD E,JR Rizalia Klausmeyer Baylor University Waco,TX 2013 Pearson Education,Inc. ALWAYS LEARNING PEARSON
© 2013 Pearson Education, Inc. Chapter 2 Lecture Organic Chemistry, 8 th Edition L. G. Wade, Jr. Structure and Properties of Organic Molecules © 2013 Pearson Education, Inc. Rizalia Klausmeyer Baylor University Waco, TX
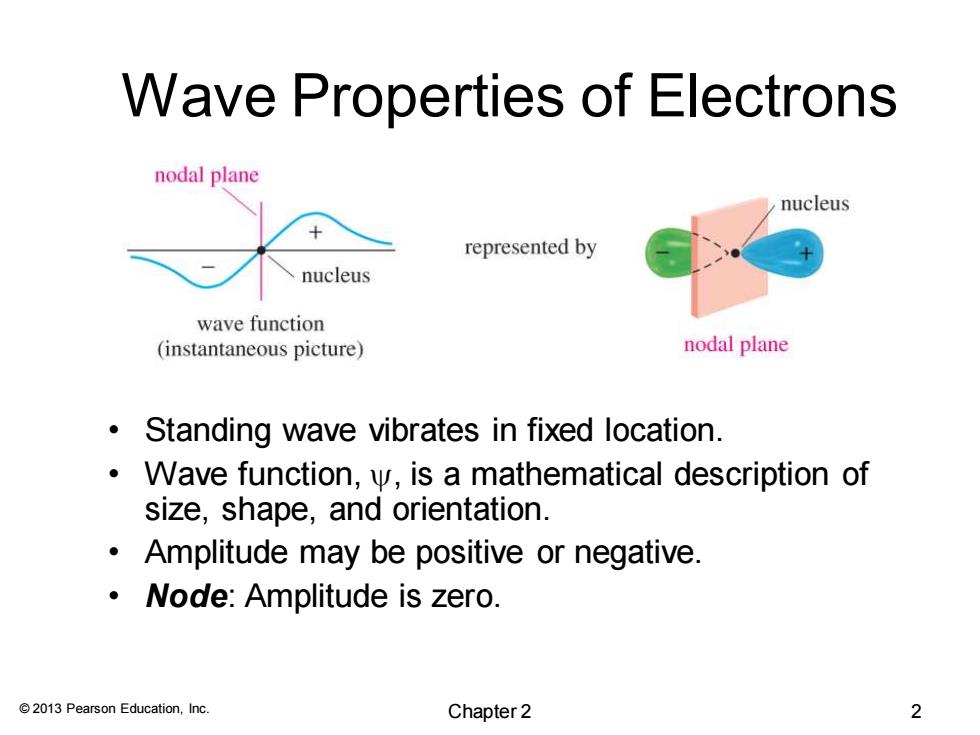
Wave Properties of Electrons nodal plane nucleus represented by nucleus wave function (instantaneous picture) nodal plane Standing wave vibrates in fixed location. Wave function,y,is a mathematical description of size,shape,and orientation. Amplitude may be positive or negative. Node:Amplitude is zero. 2013 Pearson Education,Inc. Chapter 2 2
© 2013 Pearson Education, Inc. Wave Properties of Electrons • Standing wave vibrates in fixed location. • Wave function, , is a mathematical description of size, shape, and orientation. • Amplitude may be positive or negative. • Node: Amplitude is zero. Chapter 2 2
Linear Combination of Atomic Orbitals Combining orbitals between two different atoms is bond formation. Combining orbitals on the same atom is hybridization. Conservation of orbitals Waves that are in phase add together. Amplitude increases. Waves that are out of phase cancel out 2013 Pearson Education,Inc. Chapter 2 3
© 2013 Pearson Education, Inc. Linear Combination of Atomic Orbitals • Combining orbitals between two different atoms is bond formation. • Combining orbitals on the same atom is hybridization. • Conservation of orbitals • Waves that are in phase add together. Amplitude increases. • Waves that are out of phase cancel out. Chapter 2 3
Sigma Bonding Electron density lies between the nuclei. A bond may be formed by s-s,p-p, s-p,or hybridized orbital overlaps. The bonding molecular orbital (MO)is lower in energy than the original atomic orbitals. The antibonding MO is higher in energy than the atomic orbitals. 2013 Pearson Education,Inc. Chapter 2 4
© 2013 Pearson Education, Inc. Sigma Bonding • Electron density lies between the nuclei. • A bond may be formed by s—s, p—p, s—p, or hybridized orbital overlaps. • The bonding molecular orbital (MO) is lower in energy than the original atomic orbitals. • The antibonding MO is higher in energy than the atomic orbitals. Chapter 2 4
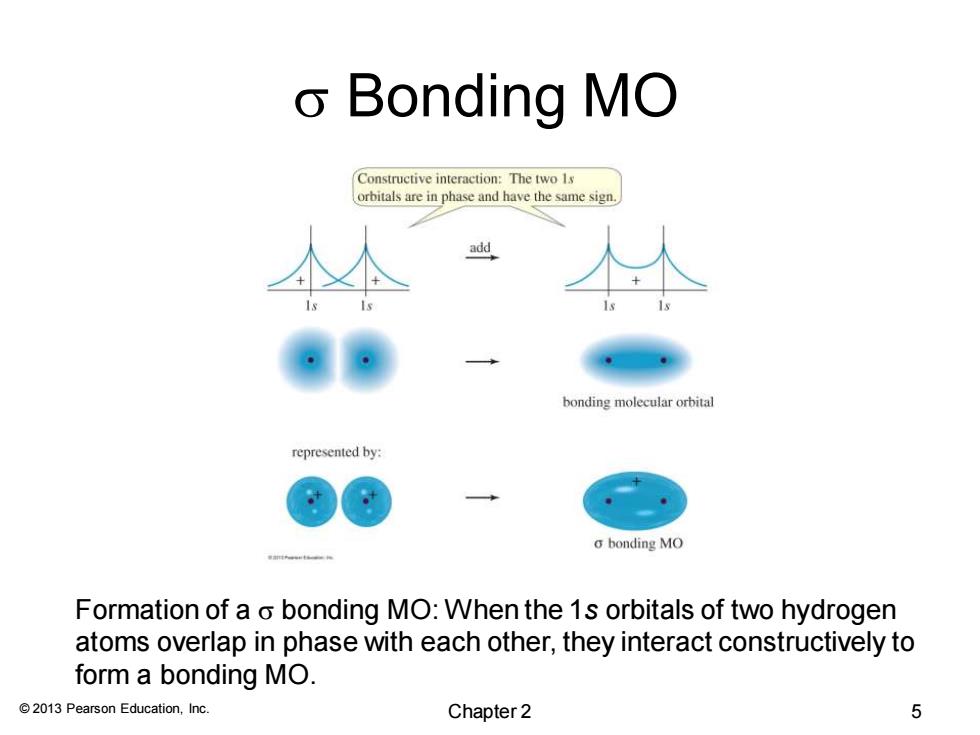
o Bonding MO Constructive interaction:The two 1s orbitals are in phase and have the same sign. add bonding molecular orbital represented by: a bonding MO Formation of a o bonding MO:When the 1s orbitals of two hydrogen atoms overlap in phase with each other,they interact constructively to form a bonding MO. 2013 Pearson Education,Inc. Chapter 2 5
© 2013 Pearson Education, Inc. s Bonding MO Formation of a s bonding MO: When the 1s orbitals of two hydrogen atoms overlap in phase with each other, they interact constructively to form a bonding MO. Chapter 2 5
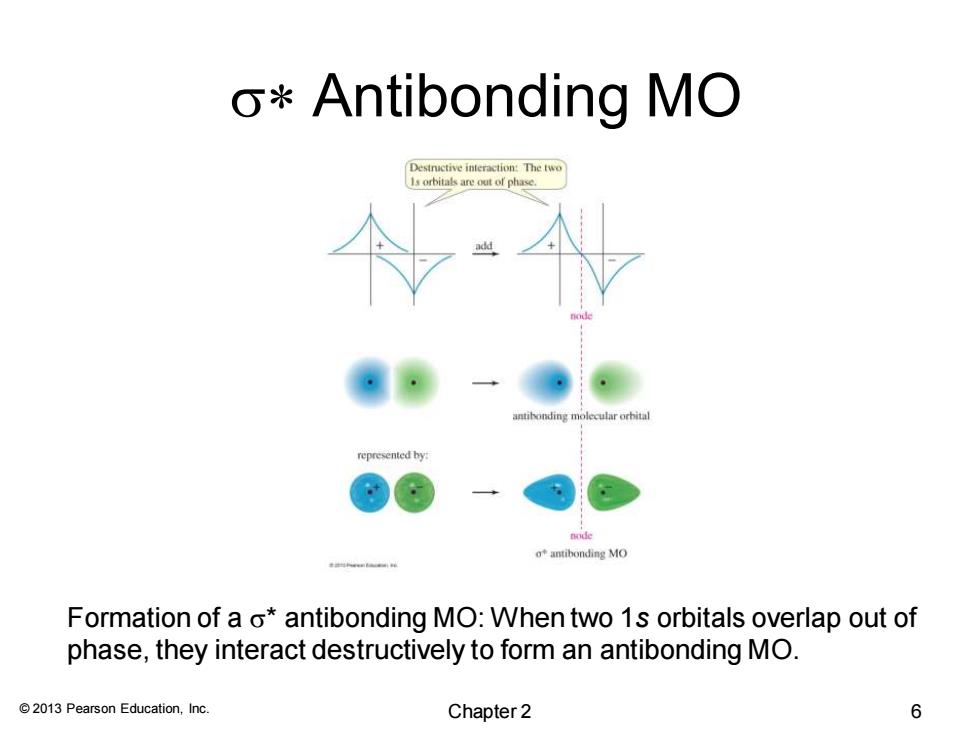
o*Antibonding MO Destructive interaction:The two 1s orbitals are out of phase. antibonding molecular orbital represented by: ot antibonding MO Formation of a o*antibonding MO:When two 1s orbitals overlap out of phase,they interact destructively to form an antibonding MO. 2013 Pearson Education,Inc. Chapter 2 6
© 2013 Pearson Education, Inc. s* Antibonding MO Formation of a s* antibonding MO: When two 1s orbitals overlap out of phase, they interact destructively to form an antibonding MO. Chapter 2 6
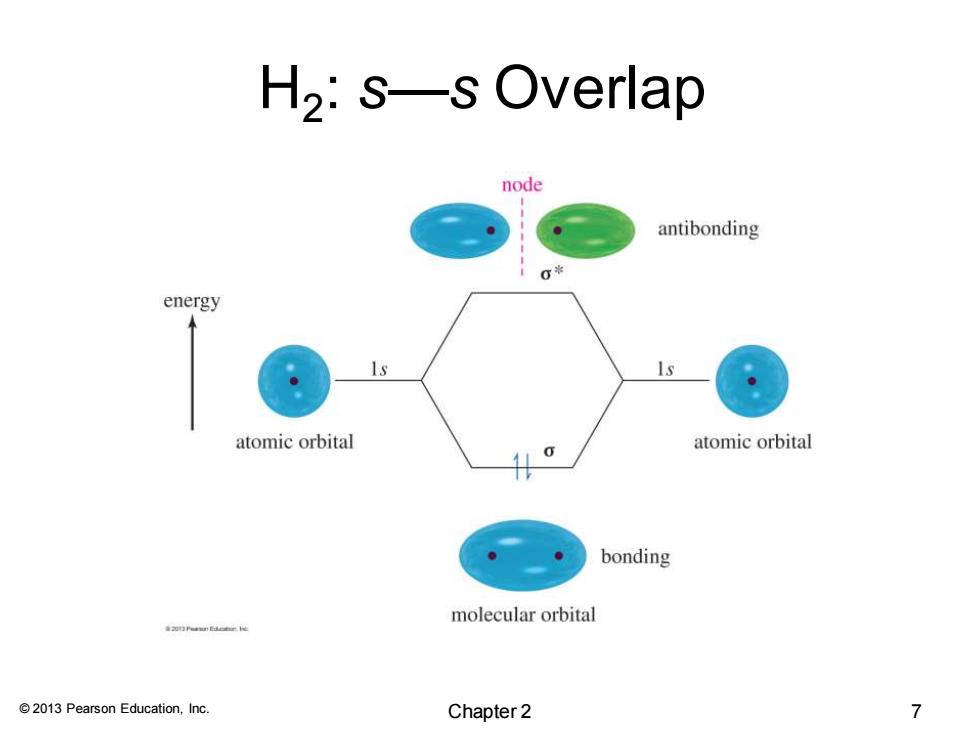
H2:s—s Overlap node antibonding 0端 energy atomic orbital atomic orbital 0 bonding molecular orbital 201 Pern Eaee 2013 Pearson Education,Inc. Chapter 2 7
© 2013 Pearson Education, Inc. H2 : s—s Overlap Chapter 2 7
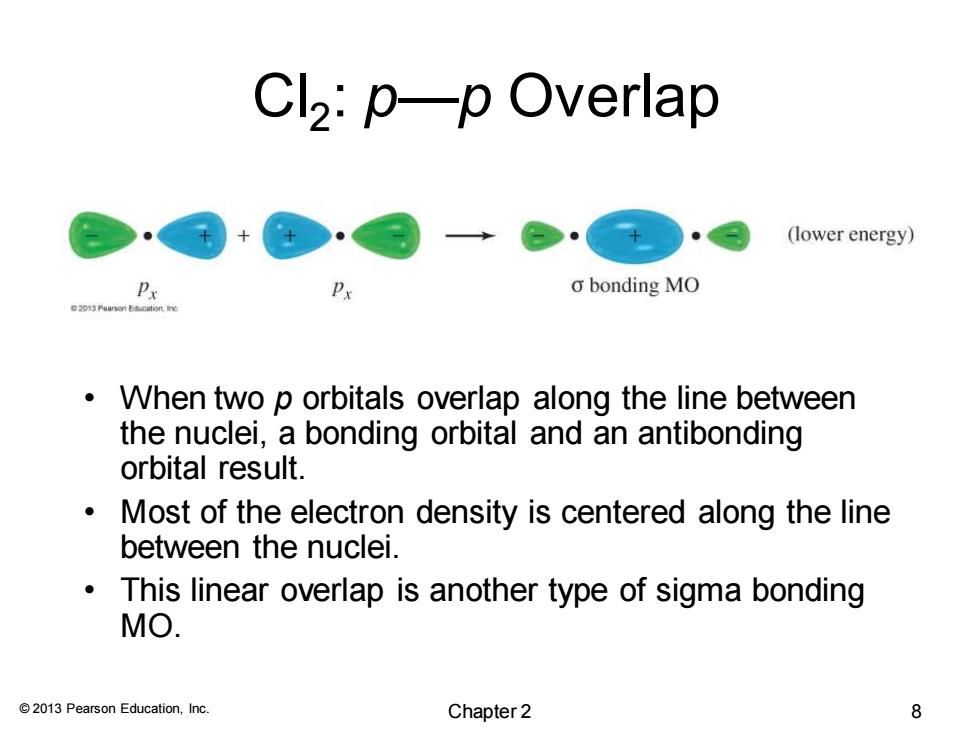
Cl2:p—p Overlap (lower energy) o bonding MO When two p orbitals overlap along the line between the nuclei,a bonding orbital and an antibonding orbital result. Most of the electron density is centered along the line between the nuclei. This linear overlap is another type of sigma bonding MO. 2013 Pearson Education,Inc. Chapter 2 8
© 2013 Pearson Education, Inc. Cl2 : p—p Overlap • When two p orbitals overlap along the line between the nuclei, a bonding orbital and an antibonding orbital result. • Most of the electron density is centered along the line between the nuclei. • This linear overlap is another type of sigma bonding MO. Chapter 2 8
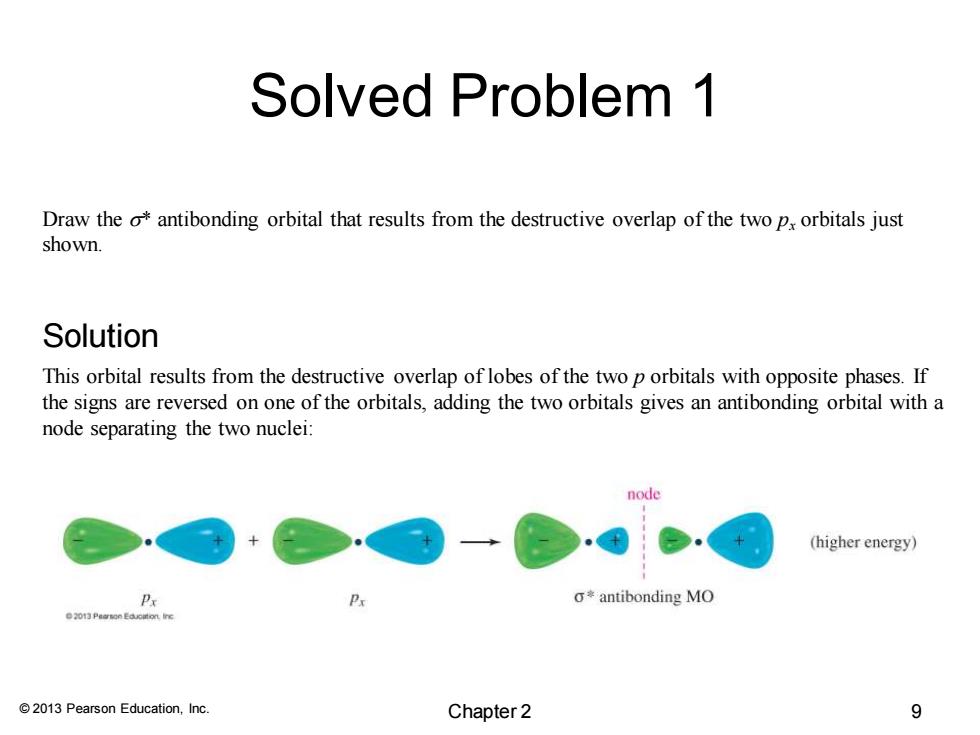
Solved Problem 1 Draw the o*antibonding orbital that results from the destructive overlap of the two px orbitals just shown. Solution This orbital results from the destructive overlap of lobes of the two p orbitals with opposite phases.If the signs are reversed on one of the orbitals,adding the two orbitals gives an antibonding orbital with a node separating the two nuclei: node (higher energy) Px g来antibonding MO 0 Pearson Eacition ire 2013 Pearson Education,Inc. Chapter 2 9
© 2013 Pearson Education, Inc. Solved Problem 1 Draw the s* antibonding orbital that results from the destructive overlap of the two px orbitals just shown. This orbital results from the destructive overlap of lobes of the two p orbitals with opposite phases. If the signs are reversed on one of the orbitals, adding the two orbitals gives an antibonding orbital with a node separating the two nuclei: Solution Chapter 2 9
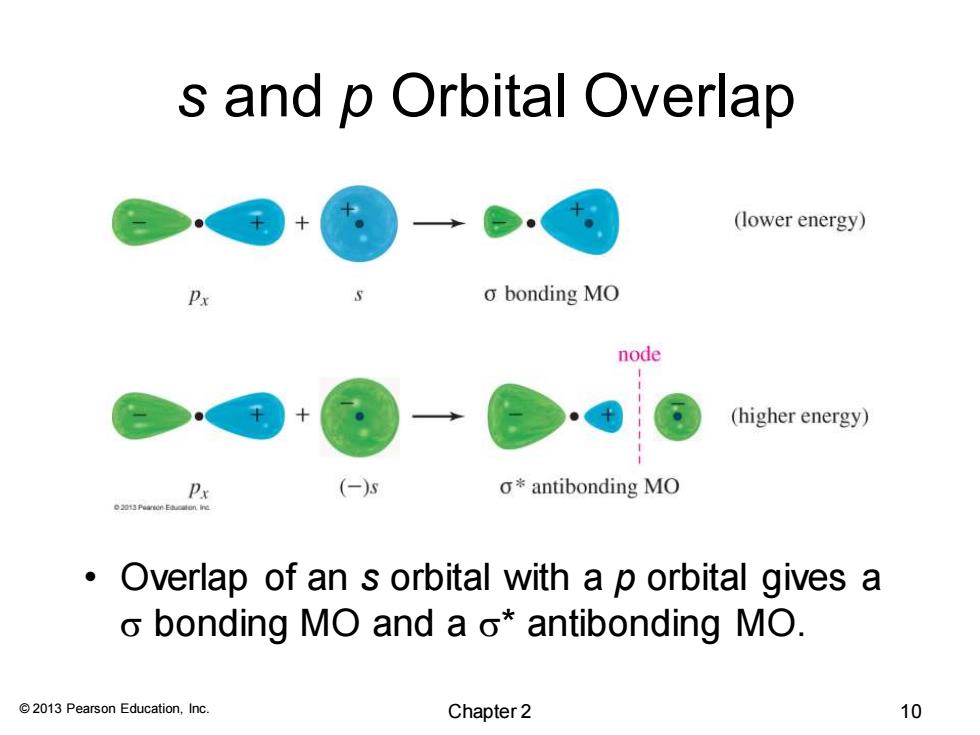
s and p Orbital Overlap (lower energy) o bonding MO node (higher energy) Px (-) o*antibonding MO 。 Overlap of an s orbital with a p orbital gives a o bonding MO and a o*antibonding MO. 2013 Pearson Education,Inc. Chapter 2 10
© 2013 Pearson Education, Inc. s and p Orbital Overlap • Overlap of an s orbital with a p orbital gives a s bonding MO and a s* antibonding MO. Chapter 2 10