
Organic Chemistry, ORGANIC 8th Edition CHEMISTRY L.G.Wade,Jr. Chapter 5 Lecture Stereochemistry G.WA D E,J R Rizalia Klausmeyer Baylor University Waco,TX 2013 Pearson Education,Inc ALWAYS LEARNING PEARSON
© 2013 Pearson Education, Inc. © 2013 Pearson Education, Inc. Chapter 5 Lecture Stereochemistry Rizalia Klausmeyer Baylor University Waco, TX Organic Chemistry, 8 th Edition L. G. Wade, Jr

Chirality right hand left hand ·“Handedness”:Right-hand glove does not fit the left hand. An object is chiral if its mirror image is different from the original object. 2013 Pearson Education,Inc. Chapter5 2
© 2013 Pearson Education, Inc. Chirality • “Handedness”: Right-hand glove does not fit the left hand. • An object is chiral if its mirror image is different from the original object. Chapter 5 2
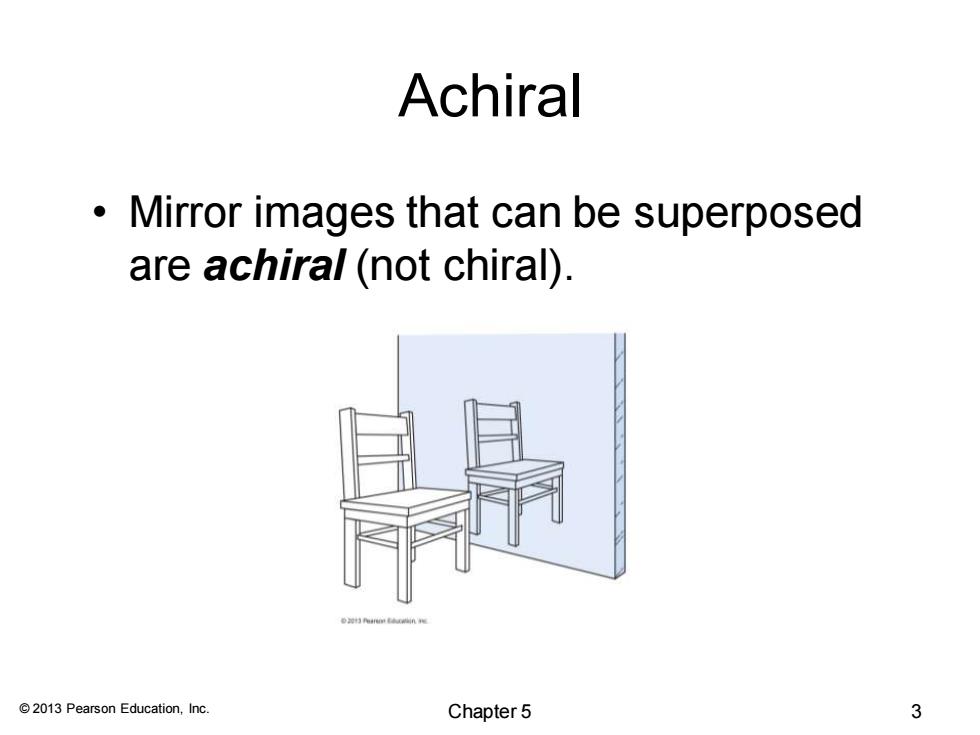
Achiral Mirror images that can be superposed are achiral(not chiral) 2013 Pearson Education,Inc. Chapter 5 3
© 2013 Pearson Education, Inc. Achiral • Mirror images that can be superposed are achiral (not chiral). Chapter 5 3
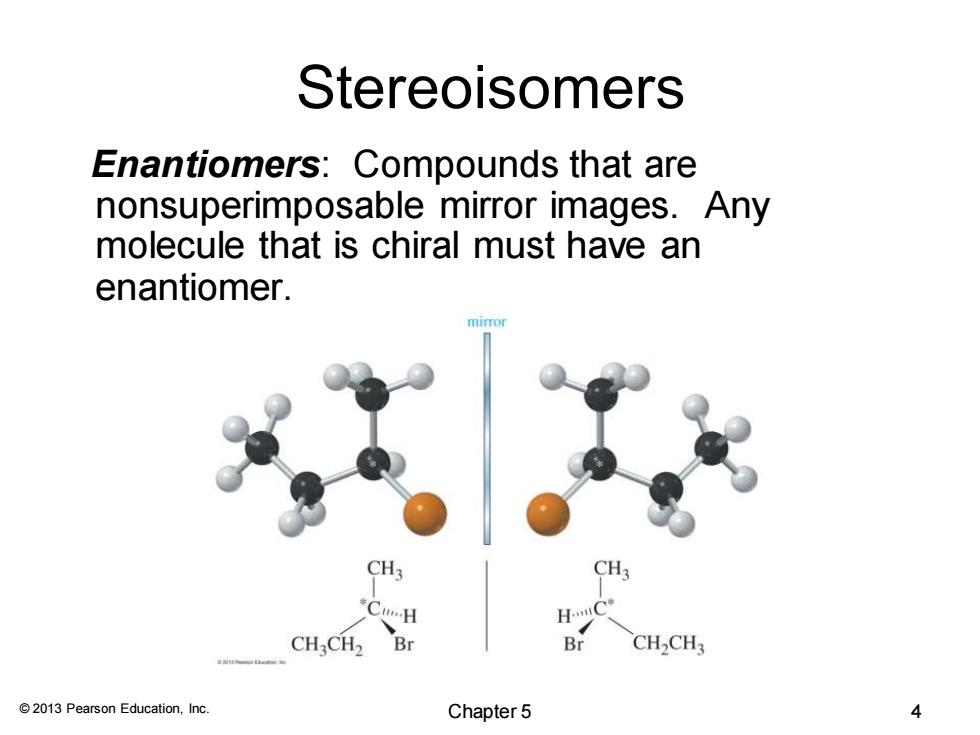
Stereoisomers Enantiomers:Compounds that are nonsuperimposable mirror images.Any molecule that is chiral must have an enantiomer. CH: CH3CH2 Br Br CH2CH3 2013 Pearson Education,Inc. Chapter 5 4
© 2013 Pearson Education, Inc. Stereoisomers Enantiomers: Compounds that are nonsuperimposable mirror images. Any molecule that is chiral must have an enantiomer. Chapter 5 4
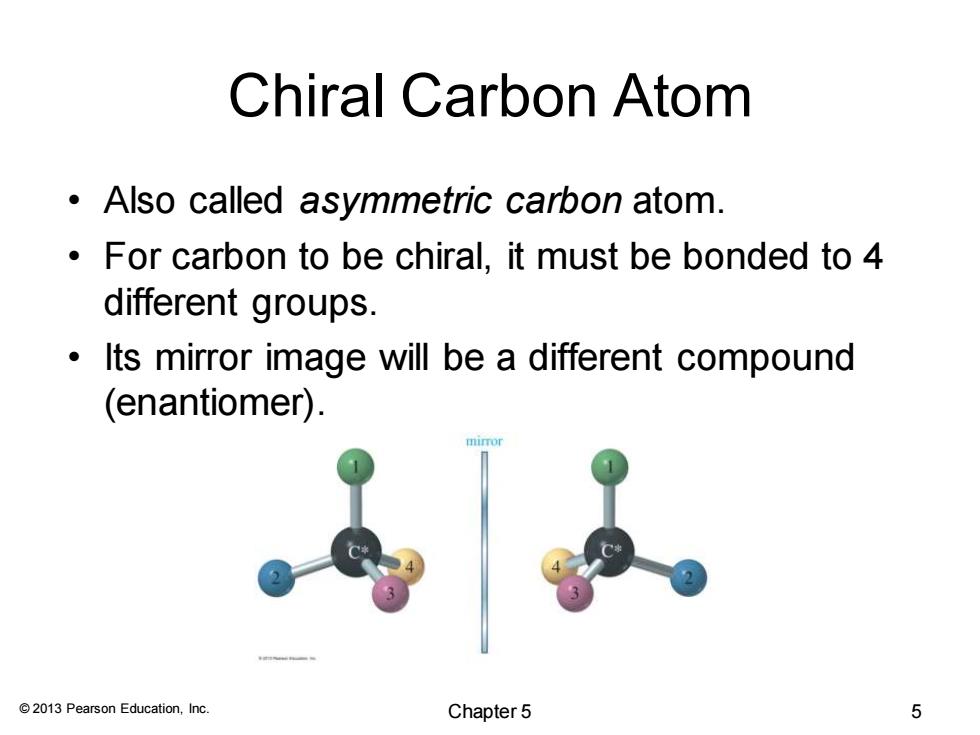
Chiral Carbon Atom Also called asymmetric carbon atom. For carbon to be chiral,it must be bonded to 4 different groups. Its mirror image will be a different compound (enantiomer). 2013 Pearson Education,Inc. Chapter 5 5
© 2013 Pearson Education, Inc. Chiral Carbon Atom • Also called asymmetric carbon atom. • For carbon to be chiral, it must be bonded to 4 different groups. • Its mirror image will be a different compound (enantiomer). Chapter 5 5
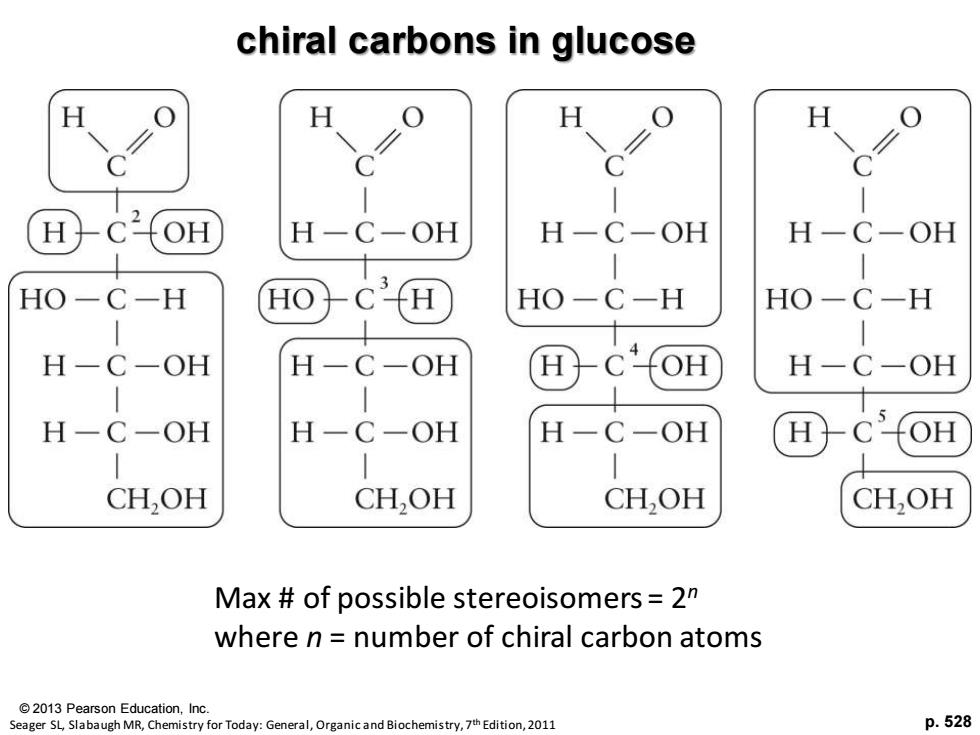
chiral carbons in glucose H H H C OH H-C-OH H-C-OH H-C-OH HO-C-H HO C HO-C-H HO-C-H | H-C-OH H-C -OH H H-C-OH H-C-OH H-C-OH H-C-OH H CH,OH CH,OH CH,OH CH,OH Max of possible stereoisomers=2 where n number of chiral carbon atoms 2013 Pearson Education,Inc. Seager SL,Slabaugh MR,Chemistry for Today:General,Organic and Biochemistry,7th Edition,2011 p.528
© 2013 Pearson Education, Inc. p. 528 chiral carbons in glucose Seager SL, Slabaugh MR, Chemistry for Today: General, Organic and Biochemistry, 7th Edition, 2011 Max # of possible stereoisomers = 2n where n = number of chiral carbon atoms
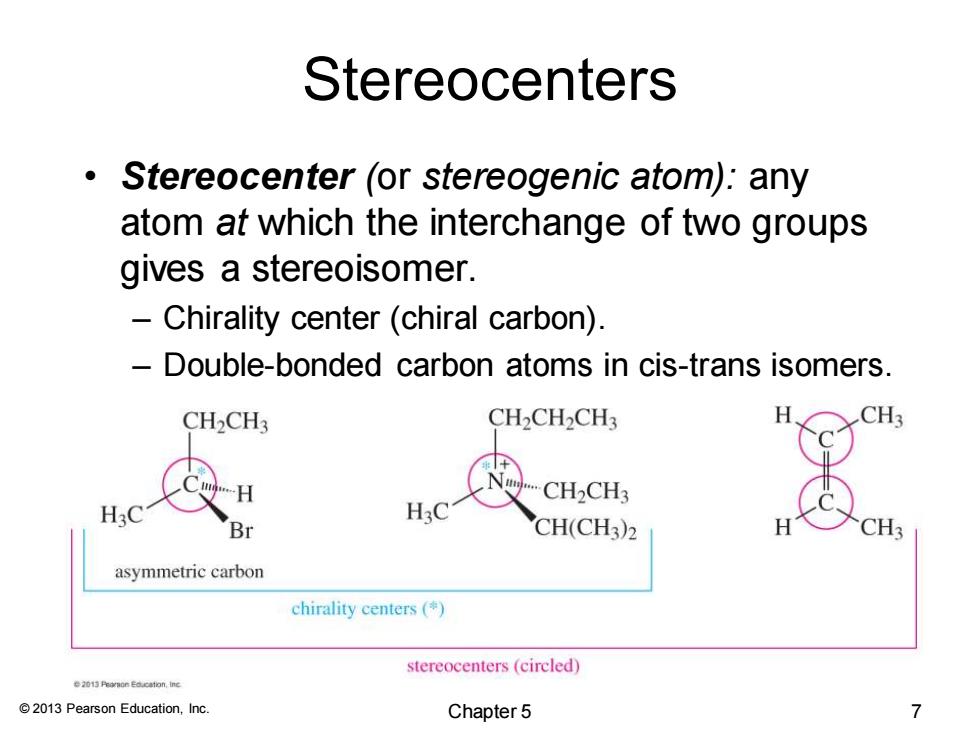
Stereocenters Stereocenter(or stereogenic atom):any atom at which the interchange of two groups gives a stereoisomer. Chirality center(chiral carbon). Double-bonded carbon atoms in cis-trans isomers. CH2CH3 CH2CH2CH3 CH3 CH2CH3 H3 Br CH(CH3)2 CH3 asymmetric carbon chirality centers ( stereocenters(circled) e20i5代on Education,e 2013 Pearson Education,Inc. Chapter 5 7
© 2013 Pearson Education, Inc. Stereocenters • Stereocenter (or stereogenic atom): any atom at which the interchange of two groups gives a stereoisomer. – Chirality center (chiral carbon). – Double-bonded carbon atoms in cis-trans isomers. Chapter 5 7
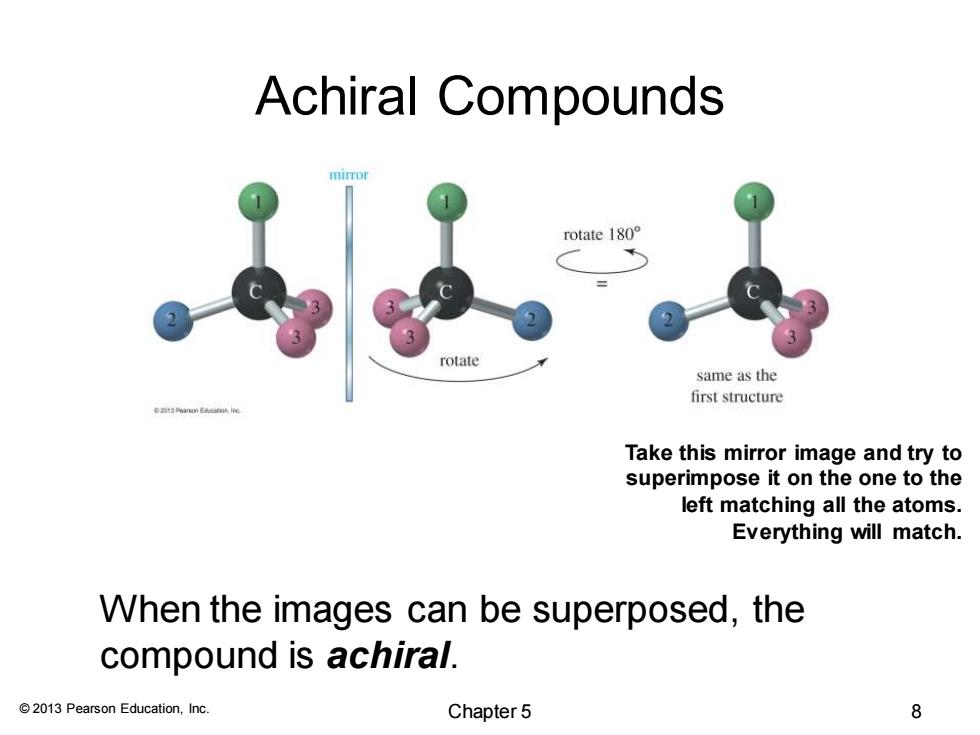
Achiral Compounds rotate 180° rotate same as the first structure Take this mirror image and try to superimpose it on the one to the left matching all the atoms. Everything will match. When the images can be superposed,the compound is achiral. 2013 Pearson Education,Inc. Chapter 5 8
© 2013 Pearson Education, Inc. Achiral Compounds Take this mirror image and try to superimpose it on the one to the left matching all the atoms. Everything will match. When the images can be superposed, the compound is achiral. Chapter 5 8
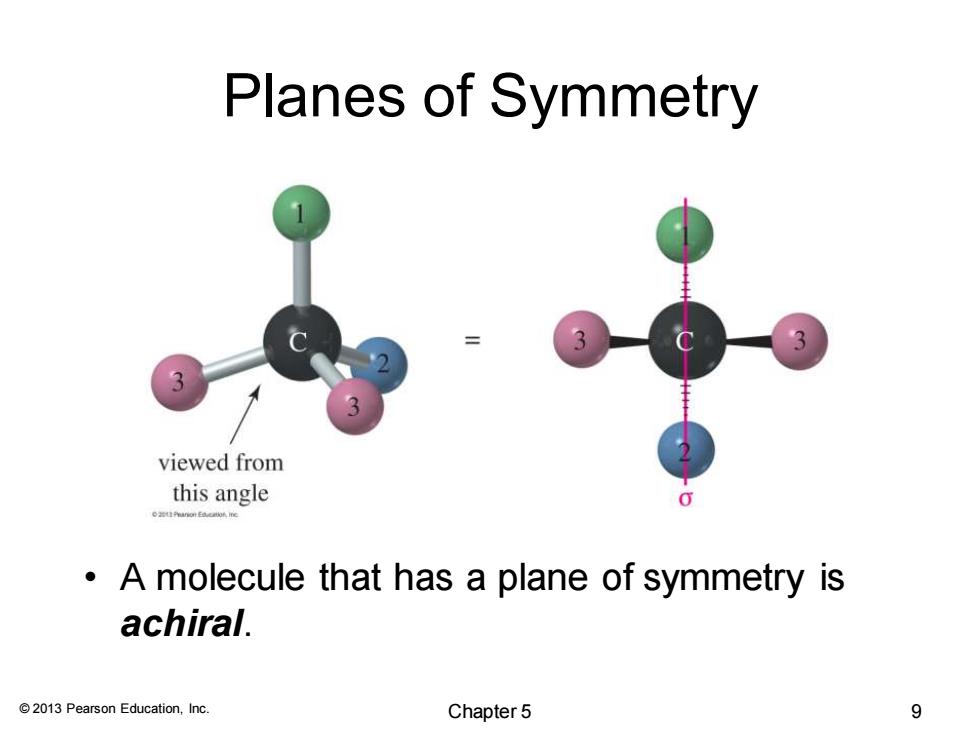
Planes of Symmetry 3 viewed from this angle 0 A molecule that has a plane of symmetry is achiral. 2013 Pearson Education,Inc. Chapter 5 9
© 2013 Pearson Education, Inc. Planes of Symmetry • A molecule that has a plane of symmetry is achiral. Chapter 5 9
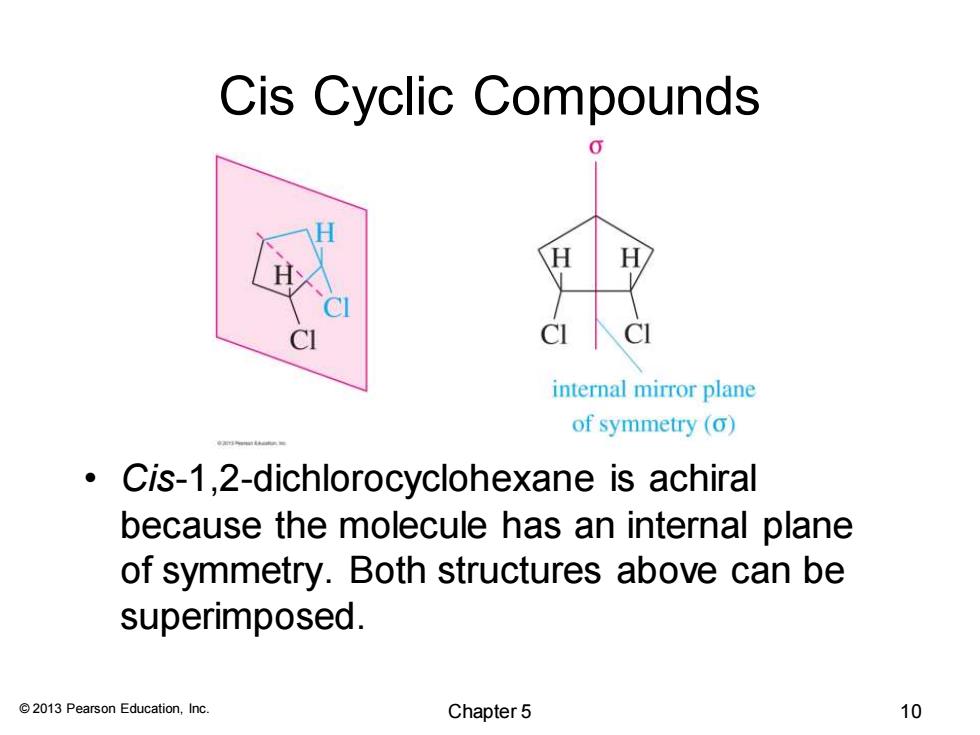
Cis Cyclic Compounds H H C internal mirror plane of symmetry (o) Cis-1,2-dichlorocyclohexane is achiral because the molecule has an internal plane of symmetry.Both structures above can be superimposed. 2013 Pearson Education,Inc. Chapter 5 10
© 2013 Pearson Education, Inc. Cis Cyclic Compounds • Cis-1,2-dichlorocyclohexane is achiral because the molecule has an internal plane of symmetry. Both structures above can be superimposed. Chapter 5 10