
Organic Chemistry,5th Edition L.G.Wade,Jr. Chapter 16 Aromatic Compounds Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2003,Prentice Hall
Chapter 16 Aromatic Compounds Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2003, Prentice Hall Organic Chemistry, 5th Edition L. G. Wade, Jr

Discovery of Benzene Isolated in 1825 by Michael Faraday who determined C:H ratio to be 1:1. Synthesized in 1834 by Eilhard Mitscherlich who determined molecular formula to be CeHe. 。 Other related compounds with low C:H ratios had a pleasant smell,so they were classified as aromatic. 3> Chapter 16
Chapter 16 2 Discovery of Benzene • Isolated in 1825 by Michael Faraday who determined C:H ratio to be 1:1. • Synthesized in 1834 by Eilhard Mitscherlich who determined molecular formula to be C6H6 . • Other related compounds with low C:H ratios had a pleasant smell, so they were classified as aromatic. =>
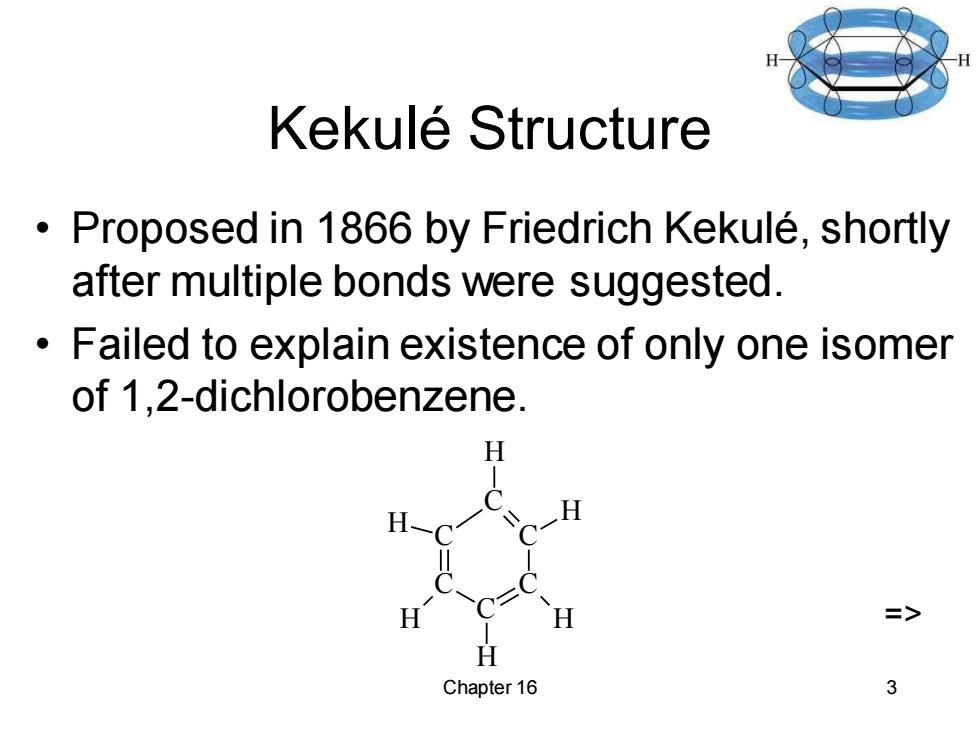
Kekule Structure Proposed in 1866 by Friedrich Kekule,shortly after multiple bonds were suggested. Failed to explain existence of only one isomer of 1,2-dichlorobenzene. H => Chapter 16 3
Chapter 16 3 Kekulé Structure • Proposed in 1866 by Friedrich Kekulé, shortly after multiple bonds were suggested. • Failed to explain existence of only one isomer of 1,2-dichlorobenzene. C C C C C C H H H H H H =>
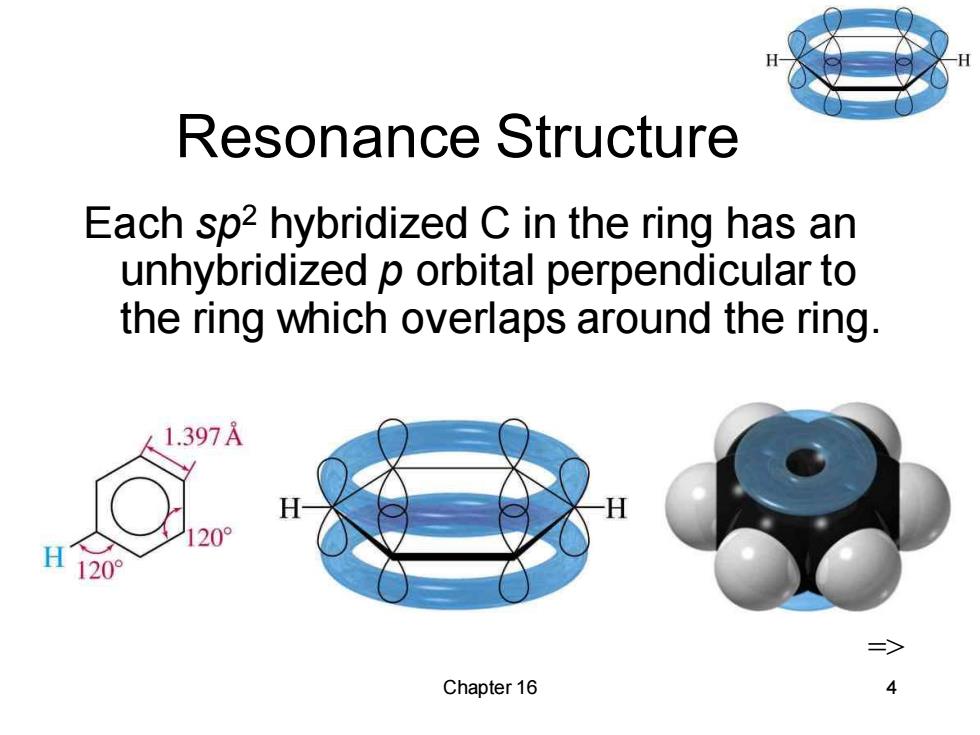
Resonance Structure Each sp2 hybridized C in the ring has an unhybridized p orbital perpendicular to the ring which overlaps around the ring. 1.397A 20 20 Chapter 16
Chapter 16 4 Resonance Structure Each sp2 hybridized C in the ring has an unhybridized p orbital perpendicular to the ring which overlaps around the ring. =>

Unusual Reactions ·Alkene+KMnO4→diol(addition) Benzene KMnO>no reaction. ·Alkene+Brz/CCl4→dibromide(addition) Benzene Br2/CCla>no reaction. With FeCla catalyst,Br2 reacts with benzene to form bromobenzene HBr (substitution!).Double bonds remain. > Chapter 16 5
Chapter 16 5 Unusual Reactions • Alkene + KMnO4 → diol (addition) Benzene + KMnO4 → no reaction. • Alkene + Br2 /CCl4 → dibromide (addition) Benzene + Br2 /CCl4 → no reaction. • With FeCl3 catalyst, Br2 reacts with benzene to form bromobenzene + HBr (substitution!). Double bonds remain. =>

Unusual Stability Hydrogenation of just one double bond in benzene is endothermic! --(-85.8 predicted) 36 kcal (-57.2 predicted) resonance energy energy 1.8 kcal resonance energy -57.4 kcal -55.4 kcal -49.8 kcal -28.6 kcal energy
Chapter 16 6 Unusual Stability Hydrogenation of just one double bond in benzene is endothermic! =>
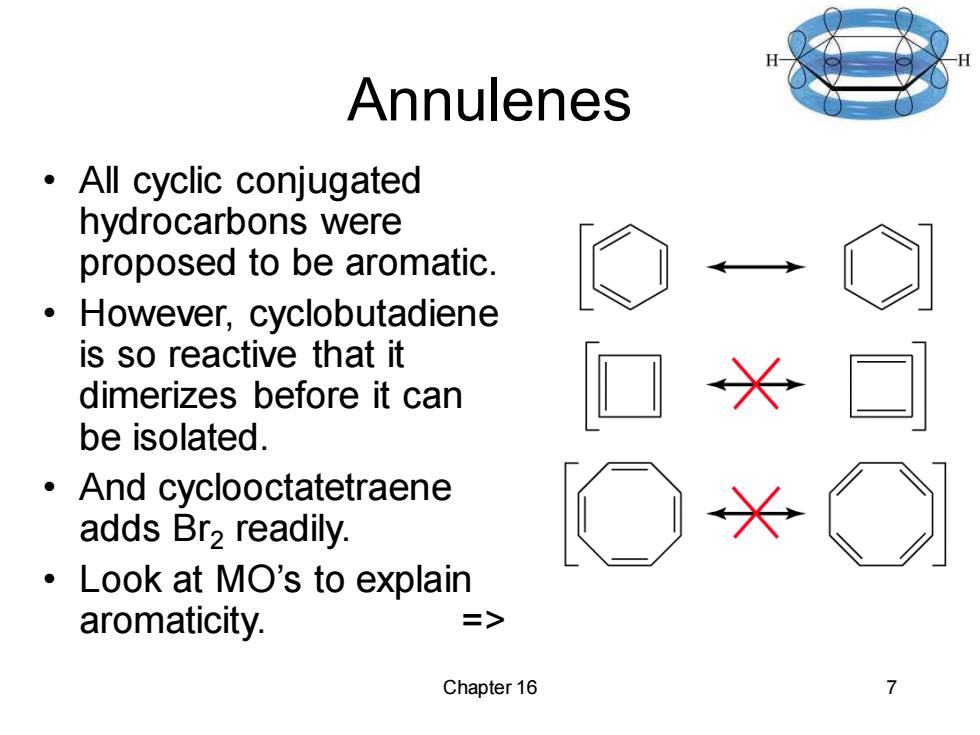
Annulenes All cyclic conjugated hydrocarbons were proposed to be aromatic. However,cyclobutadiene is so reactive that it dimerizes before it can be isolated. And cyclooctatetraene adds Br2readily. Look at MO's to explain aromaticity. => Chapter 16 >
Chapter 16 7 Annulenes • All cyclic conjugated hydrocarbons were proposed to be aromatic. • However, cyclobutadiene is so reactive that it dimerizes before it can be isolated. • And cyclooctatetraene adds Br2 readily. • Look at MO’s to explain aromaticity. =>

MO Rules for Benzene 0 Six overlapping p orbitals must form six molecular orbitals. Three will be bonding,three antibonding. Lowest energy MO will have all bonding interactions,no nodes. As energy of MO increases,the number of nodes increases. => Chapter 16 8
Chapter 16 8 MO Rules for Benzene • Six overlapping p orbitals must form six molecular orbitals. • Three will be bonding, three antibonding. • Lowest energy MO will have all bonding interactions, no nodes. • As energy of MO increases, the number of nodes increases. =>
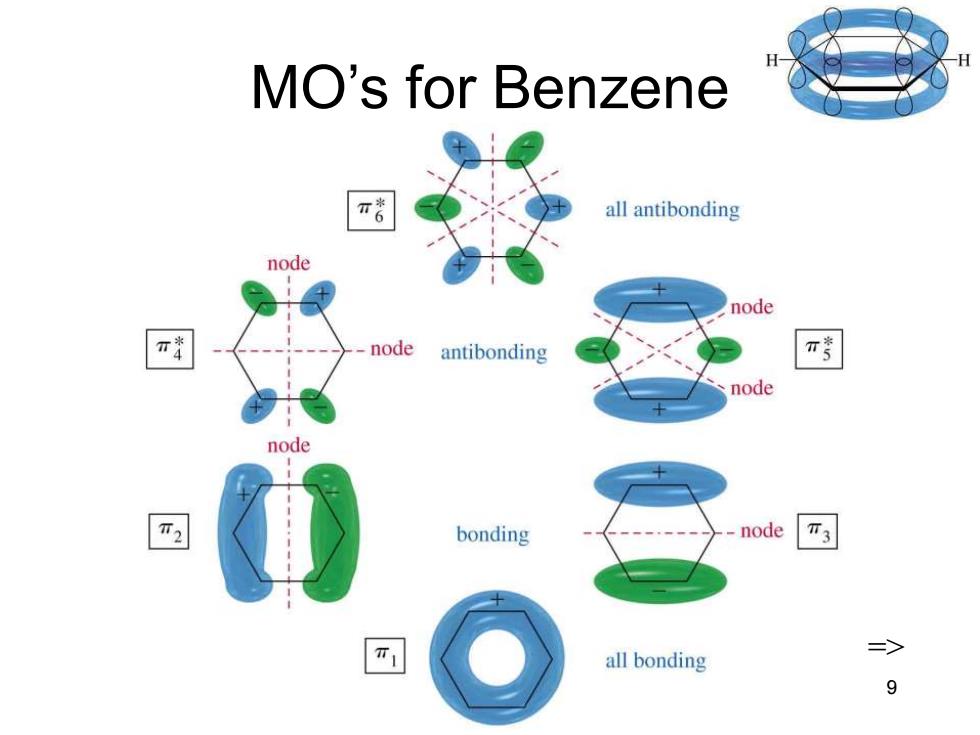
MO's for Benzene π all antibonding node node node antibonding π node node T2 bonding -node T3 all bonding 9
Chapter 16 9 MO’s for Benzene =>
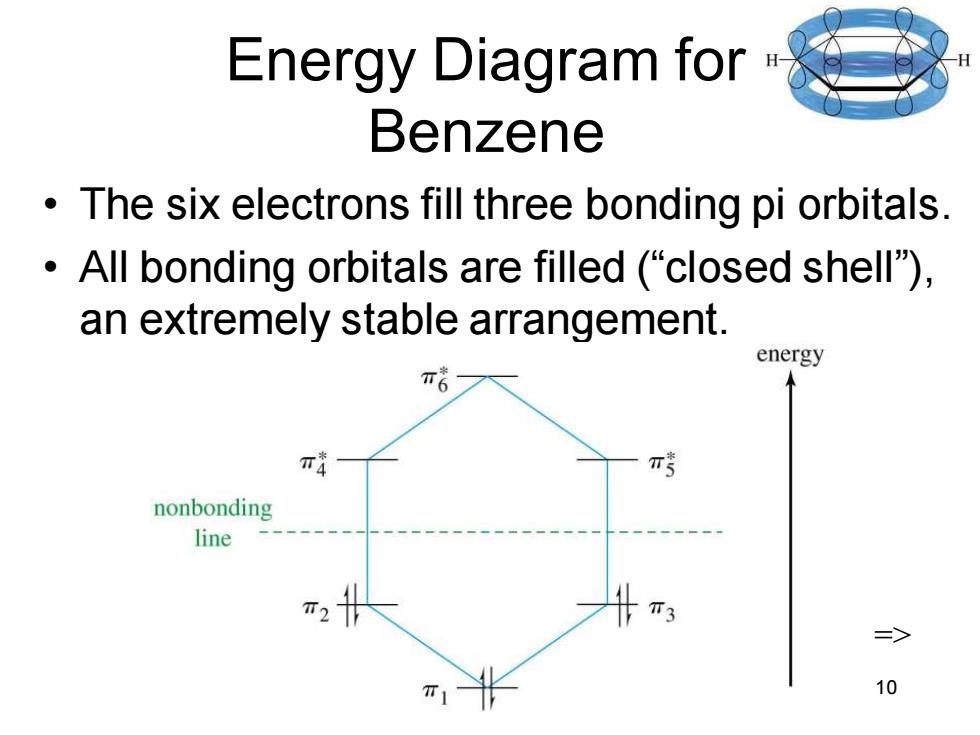
Energy Diagram for Benzene The six electrons fill three bonding pi orbitals. All bonding orbitals are filled ("closed shell). an extremely stable arrangement. energy π nonbonding line T3 10
Chapter 16 10 Energy Diagram for Benzene • The six electrons fill three bonding pi orbitals. • All bonding orbitals are filled (“closed shell”), an extremely stable arrangement. =>