
Organic Chemistry,5th Edition L.G.Wade,Jr. Chapter 3 Structure and Stereochemistry of Alkanes Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2003,Prentice Hall
Chapter 3 Structure and Stereochemistry of Alkanes Organic Chemistry, 5th Edition L. G. Wade, Jr. Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2003, Prentice Hall
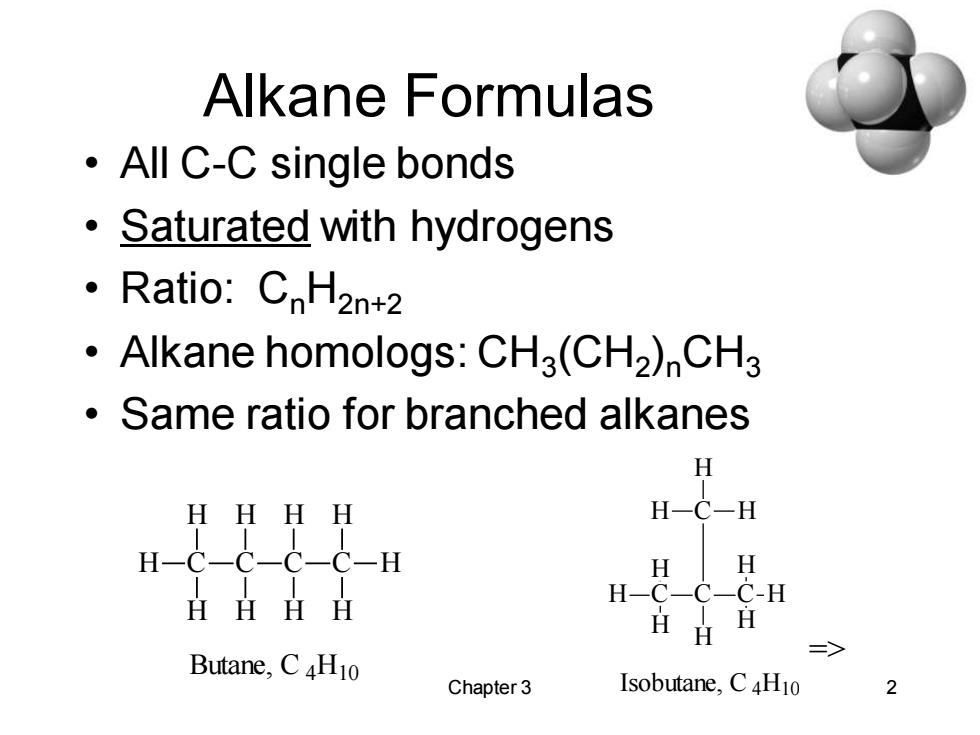
Alkane Formulas ·AllC-C single bonds Saturated with hydrogens ·Ratio::CnH2nt2 Alkane homologs:CH3(CH2)CH3 Same ratio for branched alkanes H HHHH H一C-H H-C-C-C-C-H H H HHHH H-C-C-C-H HHH Butane,CH10 Chapter 3 Isobutane,C4H10 2
Chapter 3 2 Alkane Formulas • All C-C single bonds • Saturated with hydrogens • Ratio: CnH2n+2 • Alkane homologs: CH3 (CH2 )nCH3 • Same ratio for branched alkanes H C H C H H C H H C H H H H Butane, C 4H10 => H C H C C H H H C H H H H H Isobutane, C 4H10

Common Names ·Isobutane,“isomer of butane” Isopentane,isohexane,etc.,methyl branch on next-to-last carbon in chain. Neopentane,most highly branched Five possible isomers of hexane, 18 isomers of octane and 75 for decane! Chapter 3 3
Chapter 3 3 Common Names • Isobutane, “isomer of butane” • Isopentane, isohexane, etc., methyl branch on next-to-last carbon in chain. • Neopentane, most highly branched • Five possible isomers of hexane, 18 isomers of octane and 75 for decane! =>
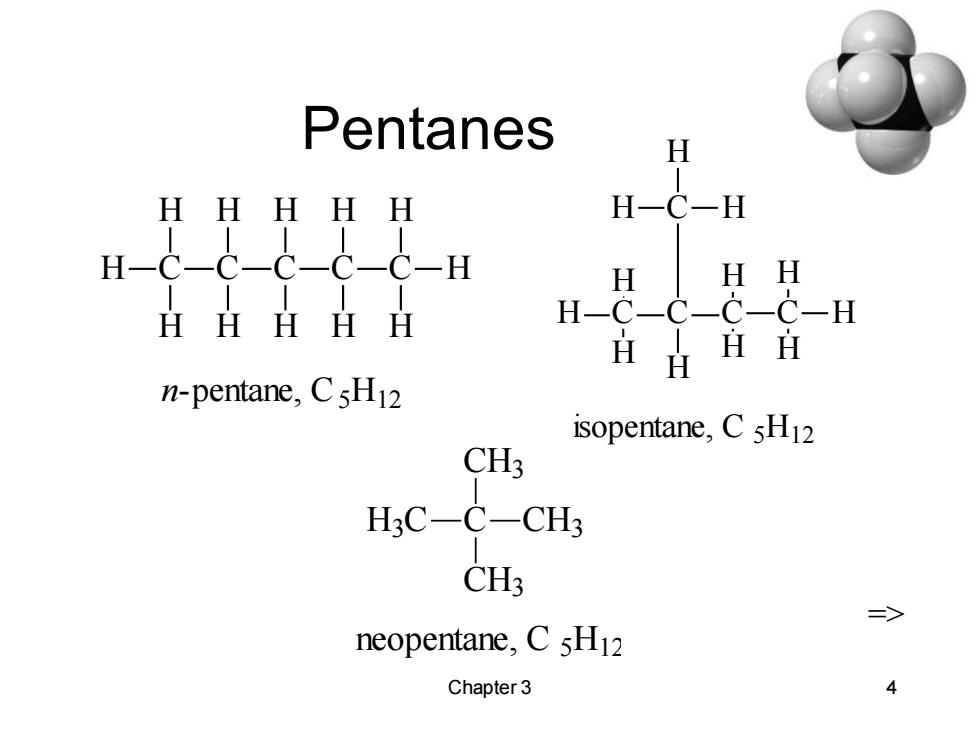
Pentanes H HHHHH H-C-H H-C-C-C-C-C-H H H H HHHHH H-C-C- -C-C-H HH n-pentane,CsH12 isopentane,CsH12 CH3 H3C-C-CH3 CH3 二> neopentane,C 5H12 Chapter 3 4
Chapter 3 4 Pentanes H C H C H H C H H C H C H H H H H n-pentane, C5 H12 H C H C C H H C H H H H H C H H H isopentane, C 5 H12 => C CH3 H3C CH3 CH3 neopentane, C 5H12

IUPAC Names Find the longest continuous carbon chain. Number the carbons,starting closest to the first branch. Name the groups attached to the chain, using the carbon number as the locator. Alphabetize substituents. Use di-,tri-,etc.,for multiples of same substituent. Chapter 3 5
Chapter 3 5 IUPAC Names • Find the longest continuous carbon chain. • Number the carbons, starting closest to the first branch. • Name the groups attached to the chain, using the carbon number as the locator. • Alphabetize substituents. • Use di-, tri-, etc., for multiples of same substituent. =>
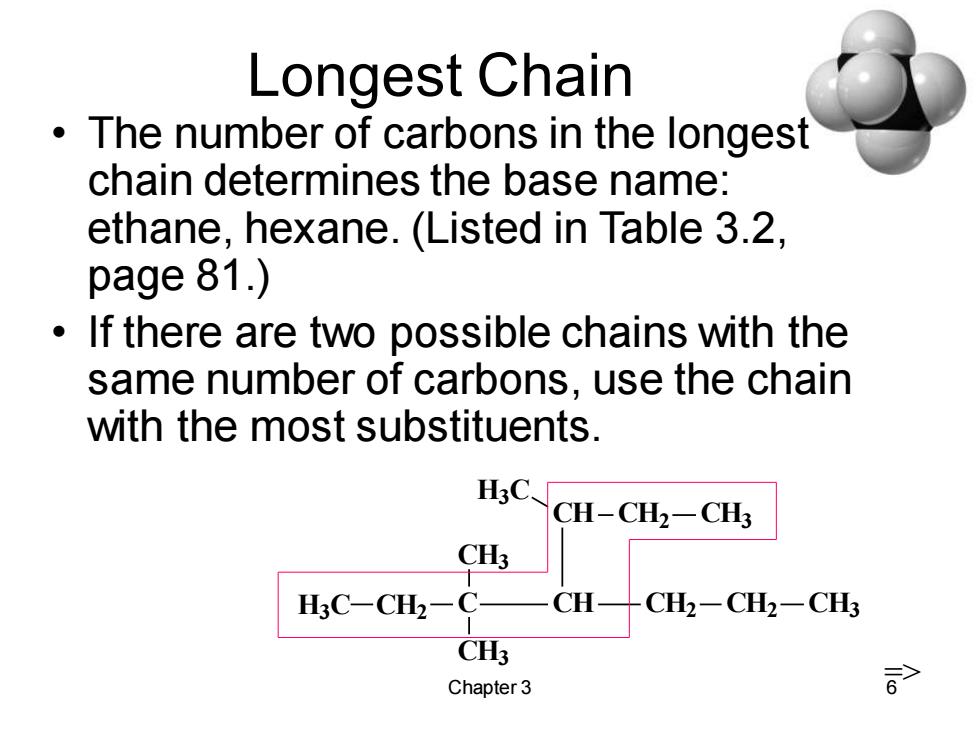
Longest Chain The number of carbons in the longest chain determines the base name: ethane,hexane.(Listed in Table 3.2, page 81.) If there are two possible chains with the same number of carbons,use the chain with the most substituents. H3C CH-CH2-CH3 CH3 H3C-CH2-C- CH CH2-CH2一CH3 CH3 Chapter 3 >
Chapter 3 6 Longest Chain • The number of carbons in the longest chain determines the base name: ethane, hexane. (Listed in Table 3.2, page 81.) • If there are two possible chains with the same number of carbons, use the chain with the most substituents. C CH3 CH2 CH3 CH CH2 CH2 CH3 CH CH2 CH3 H3C H3C =>
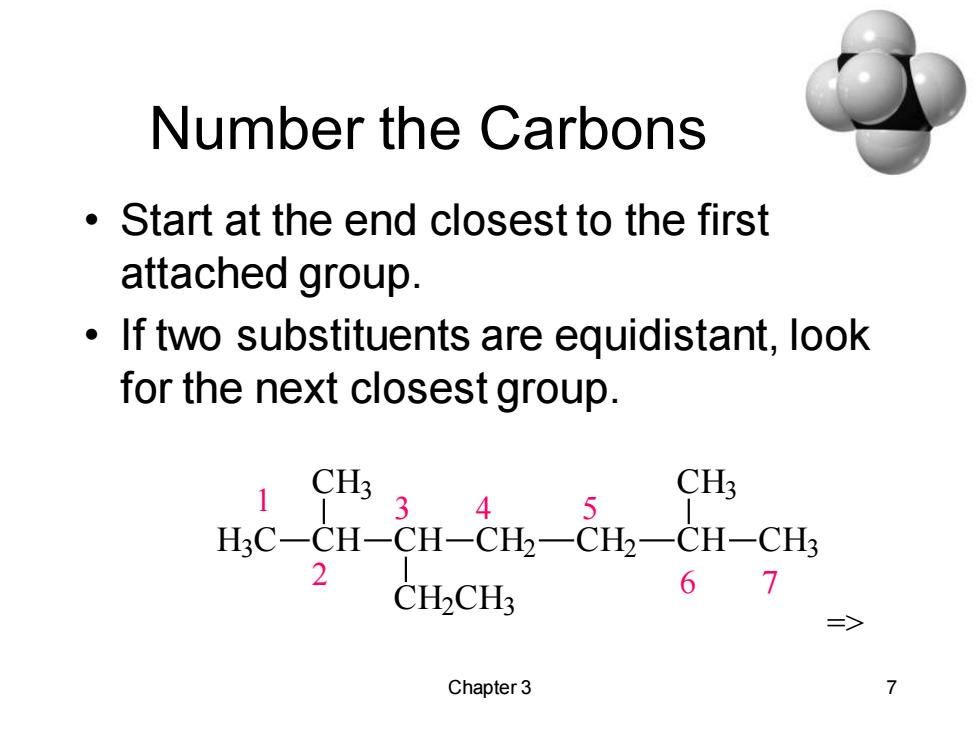
Number the Carbons Start at the end closest to the first attached group. If two substituents are equidistant,look for the next closest group. 1 CH3,4, CH3 HC-CH-CH-CH2一CH2一CH-CH3 2 CH2CH3 67 Chapter 3
Chapter 3 7 Number the Carbons • Start at the end closest to the first attached group. • If two substituents are equidistant, look for the next closest group. 1 2 3 4 5 6 7 H3 C CH CH3 CH CH2 CH3 CH2 CH2 CH CH3 CH3 =>
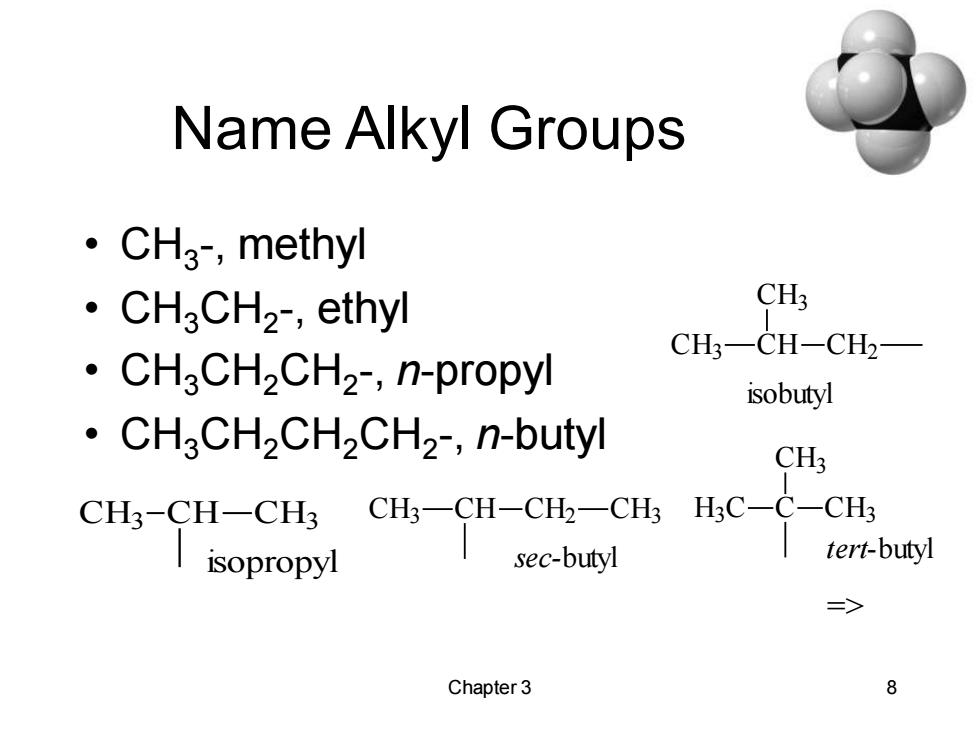
Name Alkyl Groups ·CH3,methyl ·CH3CH2,ethyl CH3 CH3一CH-CH2 ·CH3CH2CH2,n-propyl isobutyl CH3CH2CH2CH2-,n-butyl CH3 CH3-CH-CH3 CH3-CH-CH2-CH3 H;C-C-CH3 isopropyl sec-butyl tert-buty => Chapter 3 8
Chapter 3 8 Name Alkyl Groups • CH3 -, methyl • CH3CH2 -, ethyl • CH3CH2CH2 -, n-propyl • CH3CH2CH2CH2 -, n-butyl CH3 CH CH2 CH3 sec-butyl CH3 CH CH3 CH2 isobutyl CH3 CH CH3 isopropyl H3 C C CH3 CH3 tert-butyl =>
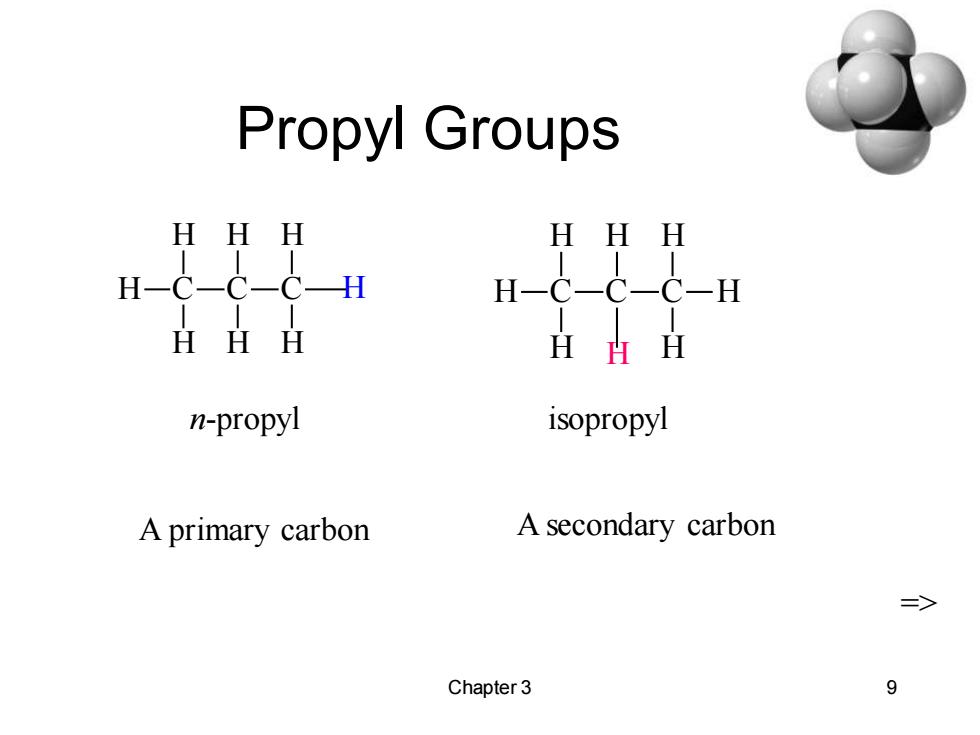
Propyl Groups HHH HH u-cccu HHH HHH n-propyl isopropyl A primary carbon A secondary carbon => Chapter 3 9
Chapter 3 9 Propyl Groups C H H H C H H C H H H n-propyl C H H H C H C H H H isopropyl H A primary carbon A secondary carbon =>
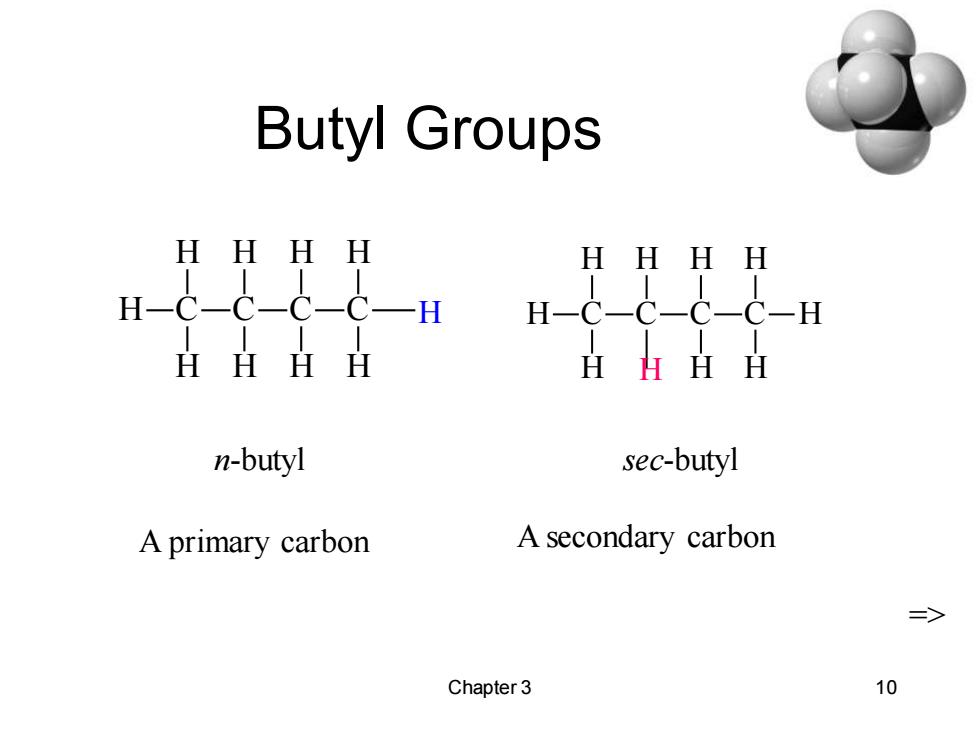
Butyl Groups HHHH HHH H HHHH HHHH n-butyl sec-butyl A primary carbon A secondary carbon => Chapter 3 10
Chapter 3 10 Butyl Groups C H H H C H C H H C H H H C H H H C H C H H H C H H n-butyl sec-butyl H H A primary carbon A secondary carbon =>