
CH3CH2CH2 Organic Chemistry,6th Edition H HH L.G.Wade,Jr. Chapter 11 Reactions of Alcohols Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2006,Prentice Hall
CH3CH2CH2 C H H Br O H H _ + Chapter 11 Reactions of Alcohols Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2006, Prentice Hall Organic Chemistry, 6th Edition L. G. Wade, Jr

CH:CH2CH2 H H Types of Alcohol Reactions Dehydration to alkene Oxidation to aldehyde,ketone Substitution to form alkyl halide ·Reduction to alkane 。Esterification ·Tosylation Williamson synthesis of ether Chapter 11 2
CH3CH2CH2 C H H Br O H H _ + Chapter 11 2 Types of Alcohol Reactions • Dehydration to alkene • Oxidation to aldehyde, ketone • Substitution to form alkyl halide • Reduction to alkane • Esterification • Tosylation • Williamson synthesis of ether =>
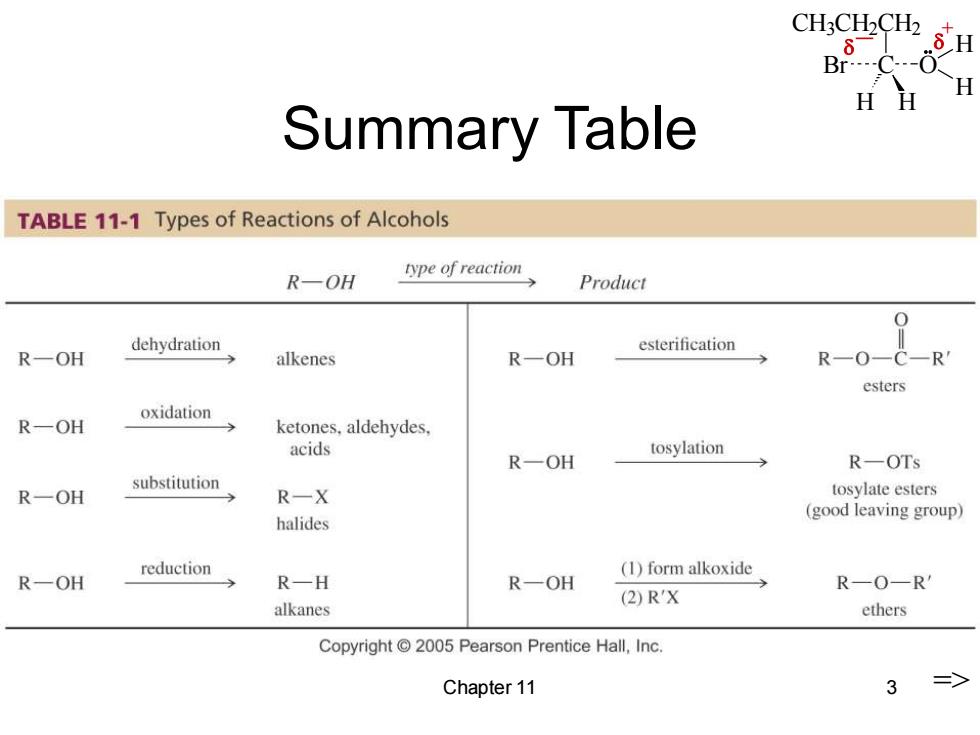
CH3CH2CH2 .8H .-0 H HH Summary Table TABLE 11-1 Types of Reactions of Alcohols R-OH type of reaction Product dehydration esterification R一OH alkenes R一OH R-0- esters oxidation R-OH ketones.aldehydes. acids tosylation R一OH R-OTs substitution R一OH R一X tosylate esters halides (good leaving group) reduction (1)form alkoxide R-OH R—H R一OH R一O-R' (2)R'X alkanes ethers Copyright 2005 Pearson Prentice Hall,Inc. Chapter 11 3 =>
CH3CH2CH2 C H H Br O H H _ + Chapter 11 3 Summary Table =>
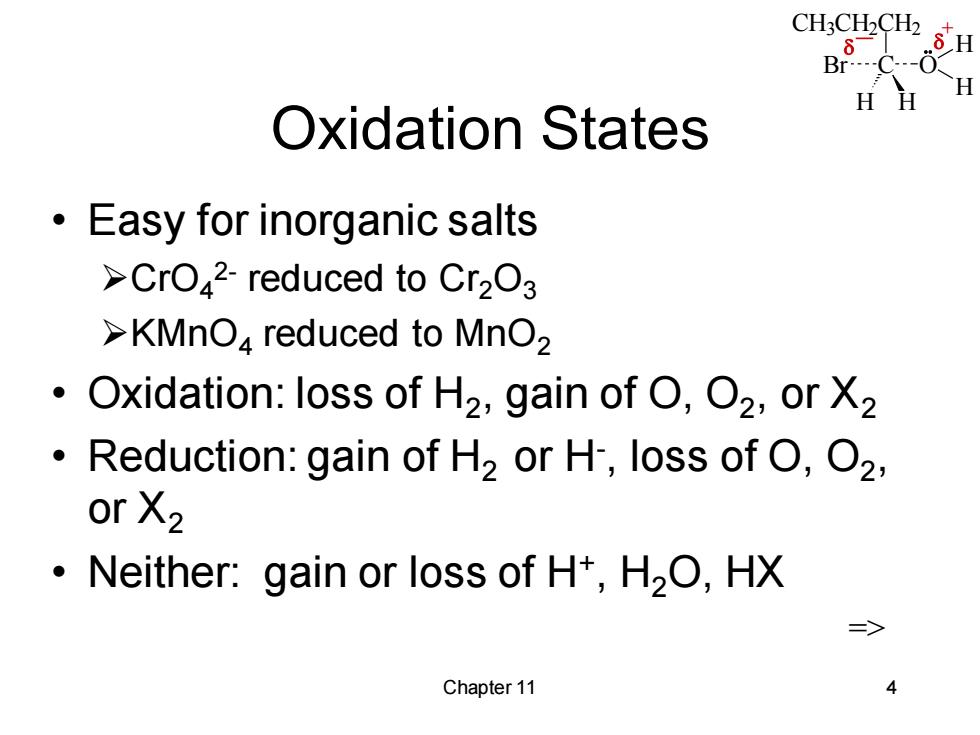
CH:CH2CH2 8 H Br---- H H Oxidation States Easy for inorganic salts >CrO42-reduced to Cr2O3 >KMnO4 reduced to MnO2 Oxidation:loss of H2,gain of O,O2,or X2 Reduction:gain of H2 or H,loss of O,O2, or X2 Neither:gain or loss of H+,H2O,HX => Chapter 11 4
CH3CH2CH2 C H H Br O H H _ + Chapter 11 4 Oxidation States • Easy for inorganic salts ➢CrO4 2- reduced to Cr2O3 ➢KMnO4 reduced to MnO2 • Oxidation: loss of H2 , gain of O, O2 , or X2 • Reduction: gain of H2 or H- , loss of O, O2 , or X2 • Neither: gain or loss of H+ , H2O, HX =>
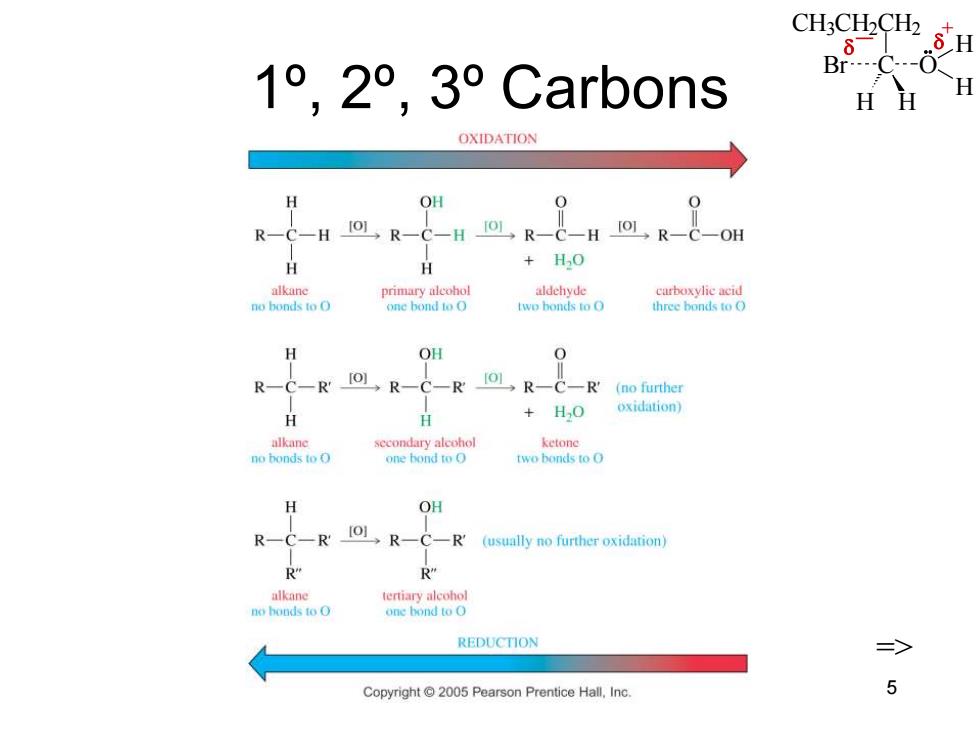
CH3CH2CH2 1°,2°,3°Carb0ns HH H OXIDATION H OH R-C-H.R-C-H IOL R-C-H [R-C-OH H H +H0 alkane primary alcohol aldehyde curboxylie acid no bonds to O one bond to O two bonds to O three bonds to O H OH R-C-R IOL R-C-R 101,R-C-R'(no further H +HO oxidation) alkane secondary alcohol ketone no bonds to O one hond to O two bonds to O H OH R-C-R R-C-R (usually no further oxidation) R" R” alkane tertiary alcohol no honds to O one hond to O REDUCTION 三> Copyright 2005 Pearson Prentice Hall,Inc 5
CH3CH2CH2 C H H Br O H H _ + Chapter 11 5 1º, 2º, 3º Carbons =>
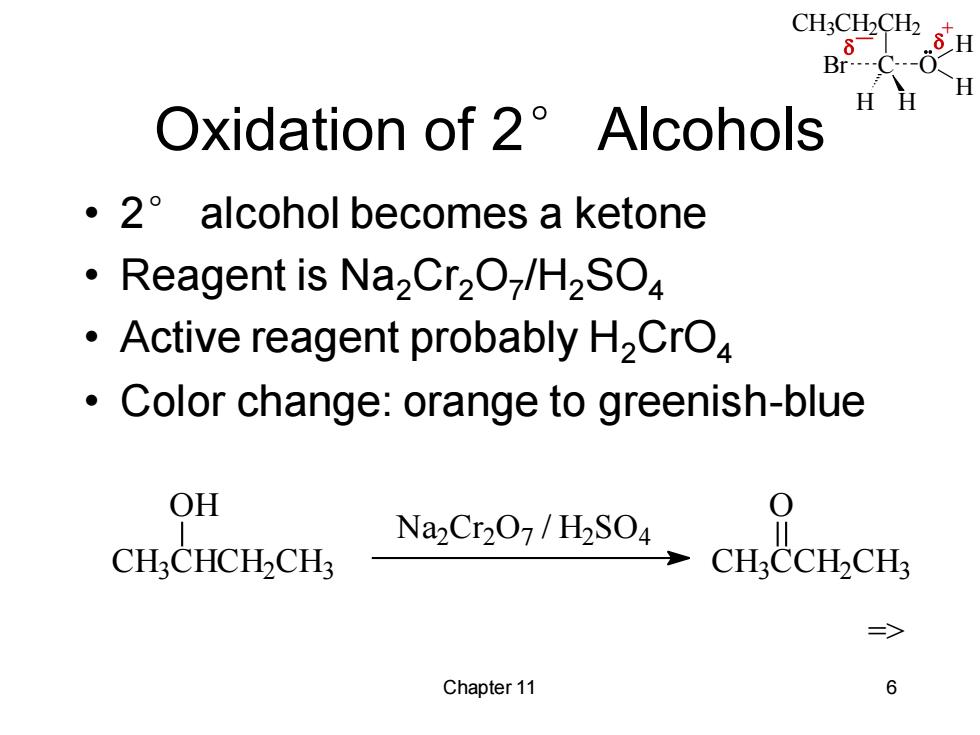
CH:CH2CH2 B8C-03 H Oxidation of 2 Alcohols ·2°alcohol becomes a ketone Reagent is Na2Cr2O/H2SO Active reagent probably H2CrO4 Color change:orange to greenish-blue OH 0 Na2Cr207/H2SO4 CH:CHCH-CH CHCCH-CH => Chapter 11 6
CH3CH2CH2 C H H Br O H H _ + Chapter 11 6 Oxidation of 2° Alcohols • 2° alcohol becomes a ketone • Reagent is Na2Cr2O7 /H2SO4 • Active reagent probably H2CrO4 • Color change: orange to greenish-blue CH3 CHCH2 CH3 OH Na2 Cr 2 O7 / H2 SO4 CH3 CCH2 CH3 O =>

CH:CH2CH2 .8H BrC-O Oxidation of 1 Alcohols H ·1°alcohol to aldehyde to carboxylic acid Difficult to stop at aldehyde Use pyridinium chlorochromate(PCC) to limit the oxidation. PCC can also be used to oxidize 2 alcohols to ketenes. OH N-H 0 CH:CH2CH2CH2 CH:CH2CH2CH Chapter 11 7
CH3CH2CH2 C H H Br O H H _ + Chapter 11 7 Oxidation of 1° Alcohols • 1° alcohol to aldehyde to carboxylic acid • Difficult to stop at aldehyde • Use pyridinium chlorochromate (PCC) to limit the oxidation. • PCC can also be used to oxidize 2° alcohols to ketones. CH3CH2CH2CH2 OH N H CrO3Cl CH3CH2CH2CH O =>

CH:CH2CH2 8 H 3 Alcohols Don't Oxidize H甘 。 Cannot lose 2 H's Basis for chromic acid test Summary of Alcohol Oxidations To Oxidize Product Reagent 2°alcohol ketone chromic acid (or PCC) 1°alcohol aldehyde PCC 1°alcohol acid chromic acid => Chapter 11 8
CH3CH2CH2 C H H Br O H H _ + Chapter 11 8 3° Alcohols Don’t Oxidize • Cannot lose 2 H’s • Basis for chromic acid test =>

CH3CH2CH2 B- .8H H HH Other Oxidation Reagents Collins reagent:Cr2O3 in pyridine Jones reagent:chromic acid in acetone ·KMnO4(strong oxidizer) ·Nitric acid(strong oxidizer) ·CuO,300°C(industrial dehydrogenation) Swern oxidation:dimethylsulfoxide,with oxalyl chloride and hindered base, oxidizes 2 alcohols to ketones and 1 alcohols to aldehydes. 士>
CH3CH2CH2 C H H Br O H H _ + Chapter 11 9 Other Oxidation Reagents • Collins reagent: Cr2O3 in pyridine • Jones reagent: chromic acid in acetone • KMnO4 (strong oxidizer) • Nitric acid (strong oxidizer) • CuO, 300°C (industrial dehydrogenation) • Swern oxidation: dimethylsulfoxide, with oxalyl chloride and hindered base, oxidizes 2 alcohols to ketones and 1 alcohols to aldehydes. =>

CH3CHCH2 Biological Oxidation Br H H Catalyzed by ADH,alcohol dehydrogenase. Oxidizing agent is NAD+,nicotinamide adenine dinucleotide. Ethanol oxidizes to acetaldehyde,then acetic acid,a normal metabolite. Methanol oxidizes to formaldehyde,then formic acid,more toxic than methanol. Ethylene glycol oxidizes to oxalic acid,toxic. Treatment for poisoning is excess ethanol. => Chapter 11 10
CH3CH2CH2 C H H Br O H H _ + Chapter 11 10 Biological Oxidation • Catalyzed by ADH, alcohol dehydrogenase. • Oxidizing agent is NAD+ , nicotinamide adenine dinucleotide. • Ethanol oxidizes to acetaldehyde, then acetic acid, a normal metabolite. • Methanol oxidizes to formaldehyde, then formic acid, more toxic than methanol. • Ethylene glycol oxidizes to oxalic acid, toxic. • Treatment for poisoning is excess ethanol. =>