
Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 15 Conjugated Systems, Orbital Symmetry,and Ultraviolet Spectroscopy Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2006,Prentice Hall
Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2006, Prentice Hall Organic Chemistry, 6th Edition L. G. Wade, Jr
Definitions Conjugated double bonds are separated by one single bond.Example:1,3-pentadiene. Isolated double bonds are separated by two or more single bonds.Example:1,4- pentadiene. Cumulated double bonds are on adjacent carbons.Example:1,2-pentadiene. => Chaper 15
Chaper 15 2 Definitions • Conjugated double bonds are separated by one single bond. Example: 1,3-pentadiene. • Isolated double bonds are separated by two or more single bonds. Example: 1,4- pentadiene. • Cumulated double bonds are on adjacent carbons. Example: 1,2-pentadiene. =>
Stabilities of Dienes Heat of hydrogenation for 1,4-pentadiene is -252 kJ/mol,about twice that of 1-pentene. ·△Hfor1-pentene is-126 kJ/mol and for trans-2-pentene is -116 kJ/mol,so expect -242 kJ/mol for trans-1,3-pentadiene. ·Actual△His-225kJ/mol,so the conjugated diene is more stable. Difference,-242 kJ(-225 kJ)=17 kJ/mol,is the resonance energy. => Chaper 15 3
Chaper 15 3 Stabilities of Dienes • Heat of hydrogenation for 1,4-pentadiene is –252 kJ/mol, about twice that of 1-pentene. • H for 1-pentene is -126 kJ/mol and for trans-2-pentene is -116 kJ/mol, so expect -242 kJ/mol for trans-1,3-pentadiene. • Actual H is -225 kJ/mol, so the conjugated diene is more stable. • Difference, -242 kJ (-225 kJ) = 17 kJ/mol, is the resonance energy. =>
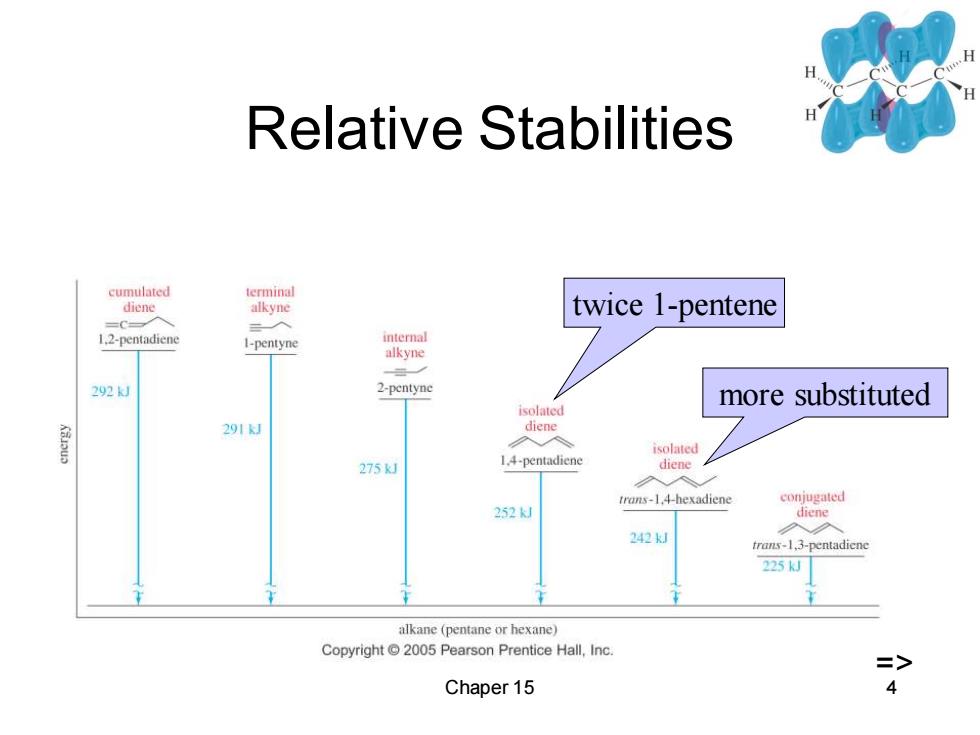
Relative Stabilities cumulated terminal diene alkyne twice 1-pentene 1.2-pentadiene I-pentyne internal alkyne 292 2-pentyne more substituted isolated 291J dlene 入 isolated 275kJ 1.4-pentadiene diene 入 trans-1.4-hexadiene conjugated 252k diene 242k」 trans-1,3-pentadicne 225kJ alkane (pentane or hexane) Copyright 2005 Pearson Prentice Hall,Inc. => Chaper 15 4
Chaper 15 4 Relative Stabilities twice 1-pentene more substituted =>

Structure of 1,3-Butadiene Most stable conformation is planar. Single bond is shorter than 1.54 A. Electrons are delocalized over molecule. => small amount of overlap partial double bond 1.34A H 1.34A Copyright2005 Pearson Prentice Hall,Inc
Chaper 15 5 Structure of 1,3-Butadiene • Most stable conformation is planar. • Single bond is shorter than 1.54 Å. • Electrons are delocalized over molecule. =>
Constructing Molecular Orbitals Pi molecular orbitals are the sideways overlap of p orbitals. p orbitals have 2 lobes.Plus (+and minus (-)indicate the opposite phases of the wave function,not electrical charge. When lobes overlap constructively,(and +or-and -)a bonding MO is formed. When and-lobes overlap,waves cancel out and a node forms;antibonding MO.= Chaper 15 6
Chaper 15 6 Constructing Molecular Orbitals • Pi molecular orbitals are the sideways overlap of p orbitals. • p orbitals have 2 lobes. Plus (+) and minus (-) indicate the opposite phases of the wave function, not electrical charge. • When lobes overlap constructively, (+ and +, or - and -) a bonding MO is formed. • When + and - lobes overlap, waves cancel out and a node forms; antibonding MO. =>
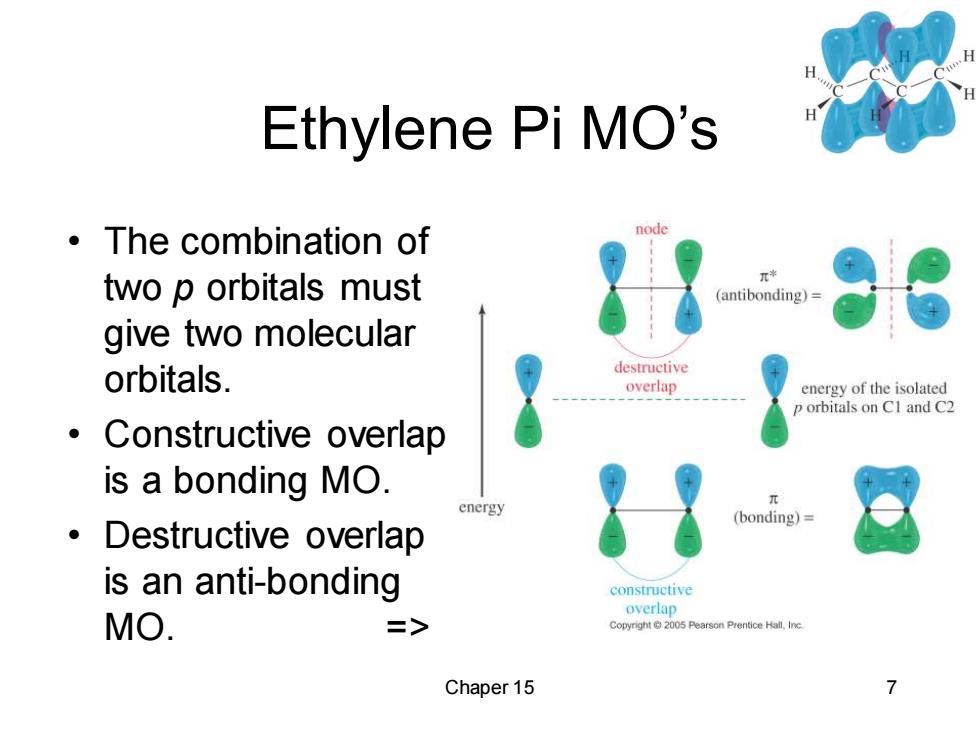
Ethylene Pi MO's ·The combination of node two p orbitals must π* (antibonding)= give two molecular orbitals. destructive overlap energy of the isolated p orbitals on CI and C2 ● Constructive overlap is a bonding MO. energy (bonding)= 。Destructive overlap is an anti-bonding constructive overlap MO. => Copyright2005 Pearson Prentice Hall.Inc Chaper 15
Chaper 15 7 Ethylene Pi MO’s • The combination of two p orbitals must give two molecular orbitals. • Constructive overlap is a bonding MO. • Destructive overlap is an anti-bonding MO. =>
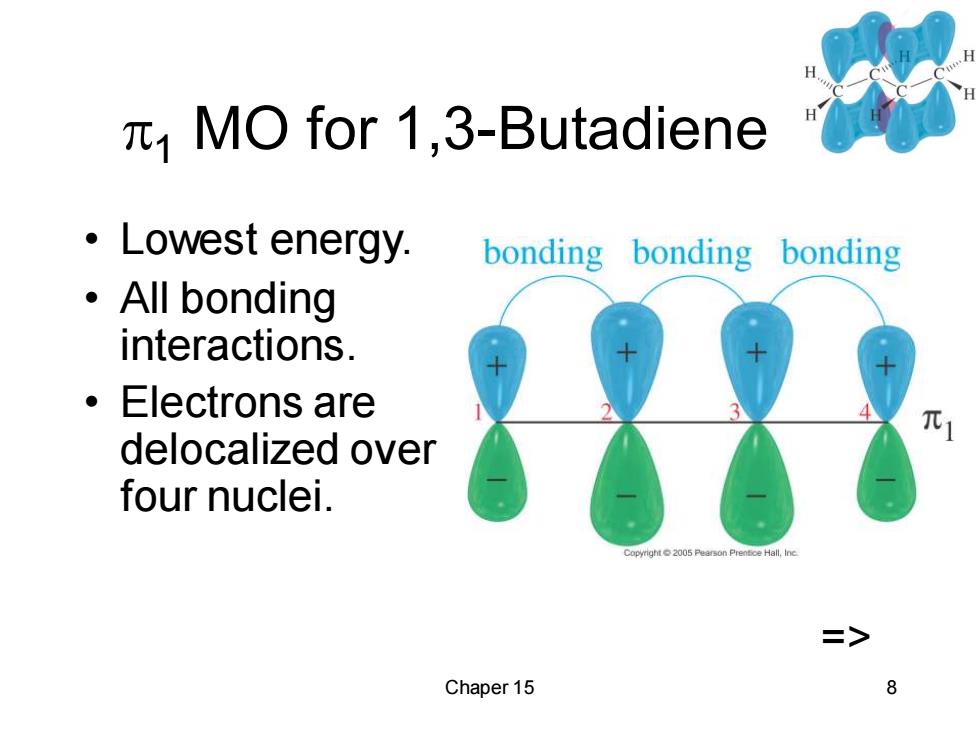
MO for 1,3-Butadiene ·Lowest energy, bonding bonding bonding ·All bonding interactions. ● Electrons are delocalized over four nuclei. Copyright2005 Peerson Prentce Hall,ine > Chaper 15 8
Chaper 15 8 1 MO for 1,3-Butadiene • Lowest energy. • All bonding interactions. • Electrons are delocalized over four nuclei. =>
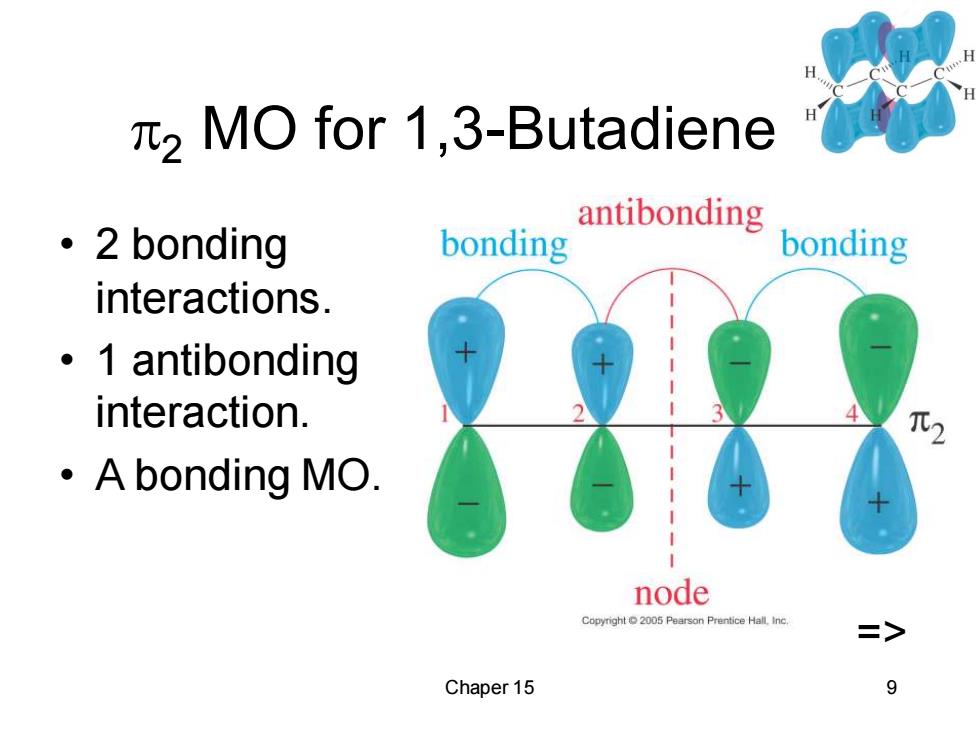
2 MO for 1,3-Butadiene 。2 bonding bonding antibonding bonding interactions. 。1 antibonding interaction. ·A bonding MO. node Copyright2005 Pearson Prentice Hall.Inc. > Chaper 15 9
Chaper 15 9 2 MO for 1,3-Butadiene • 2 bonding interactions. • 1 antibonding interaction. • A bonding MO. =>
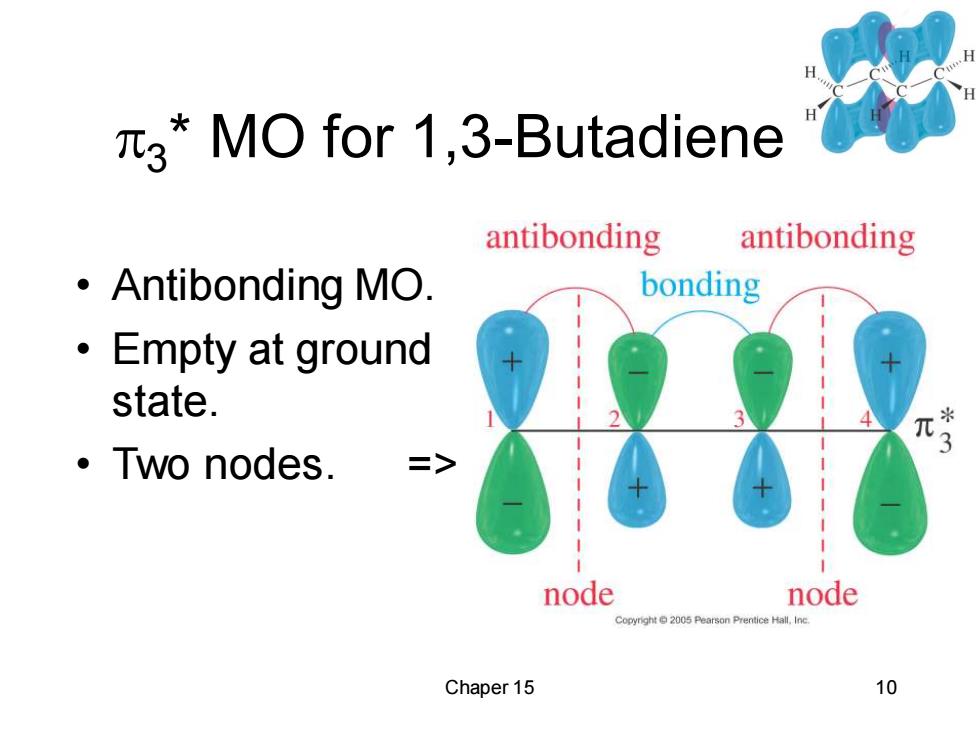
3*MO for 1,3-Butadiene antibonding antibonding ·Antibonding MO. bonding ·Empty at ground state. ·Two nodes. node node Copyright 2005 Pearson Prentice Hall.Inc Chaper 15 10
Chaper 15 10 3 * MO for 1,3-Butadiene • Antibonding MO. • Empty at ground state. • Two nodes. =>