
Organic Chemistry,5th Edition L.G.Wade,Jr. Chapter 26 Synthetic Polymers Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2003,Prentice Hall
Chapter 26 Synthetic Polymers Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2003, Prentice Hall Organic Chemistry, 5th Edition L. G. Wade, Jr
Introduction A polymer is a large molecule composed of many smaller repeating units. First synthetic polymers: >Poly(vinyl chloride)in 1838 >Polystyrene in 1839 Now,250 billion pounds produced annually,worldwide. Chapter 26
Chapter 26 2 Introduction • A polymer is a large molecule composed of many smaller repeating units. • First synthetic polymers: ➢Poly(vinyl chloride) in 1838 ➢Polystyrene in 1839 • Now, 250 billion pounds produced annually, worldwide. =>
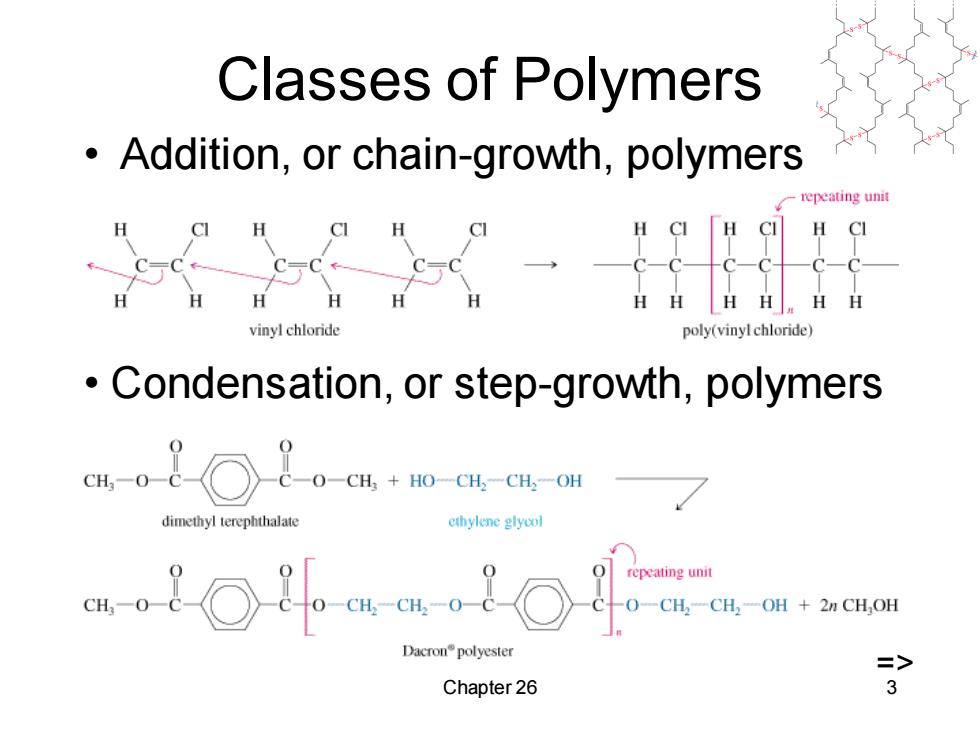
Classes of Polymers Addition,or chain-growth,polymers repeating unit H CI C- HHHH vinylchloride poly(vinyl chloride) Condensation,or step-growth,polymers dimethyl terephthalate ethylene glycol o o-o-om repeating unit Dacron°polyester Chapter 26 3
Chapter 26 3 Classes of Polymers • Addition, or chain-growth, polymers • Condensation, or step-growth, polymers =>
Addition Polymers Three kinds of intermediates: >Free radicals >Carbocations >Carbanions Examples of addition polymers: >polypropylene plastics >polystyrene foam insulation >poly(acrylonitrile)Orlon fiber >poly(methyl a-methacrylate) Plexiglas. Chapter 26
Chapter 26 4 Addition Polymers • Three kinds of intermediates: ➢Free radicals ➢Carbocations ➢Carbanions • Examples of addition polymers: ➢polypropylene plastics ➢polystyrene foam insulation ➢poly(acrylonitrile) Orlon® fiber ➢poly(methyl -methacrylate) Plexiglas ® =>
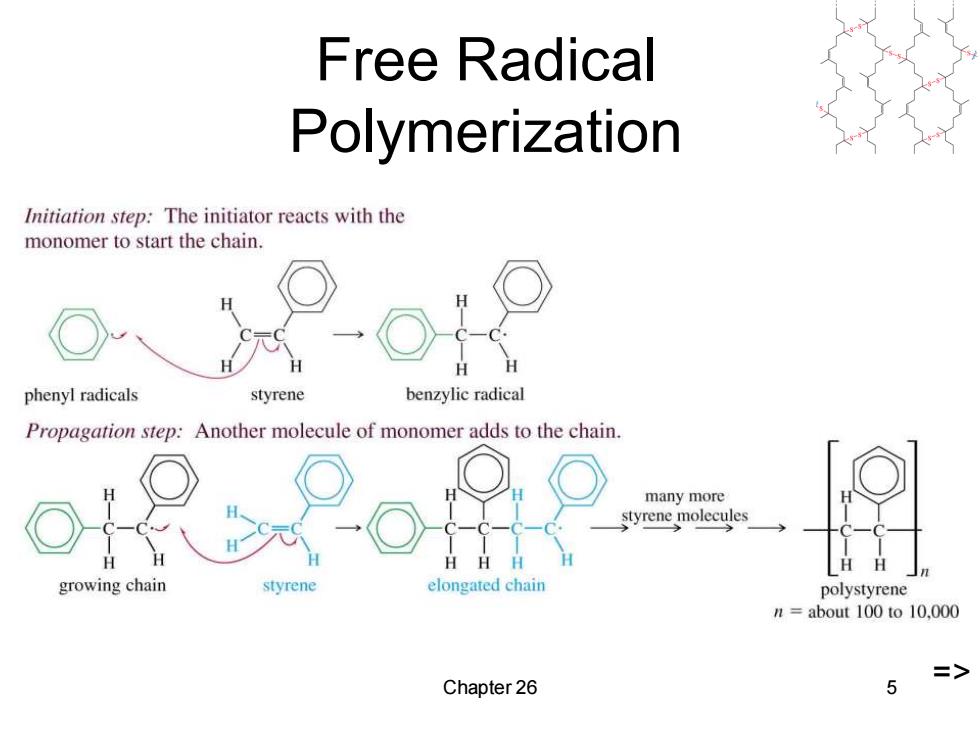
Free Radical Polymerization Initiation step:The initiator reacts with the monomer to start the chain. phenyl radicals styrene benzylic radical Propagation step:Another molecule of monomer adds to the chain. many more styrene molecules H growing chain styrene elongated chain polystyrene n=about 100 to 10.000 > Chapter 26 5
Chapter 26 5 Free Radical Polymerization =>
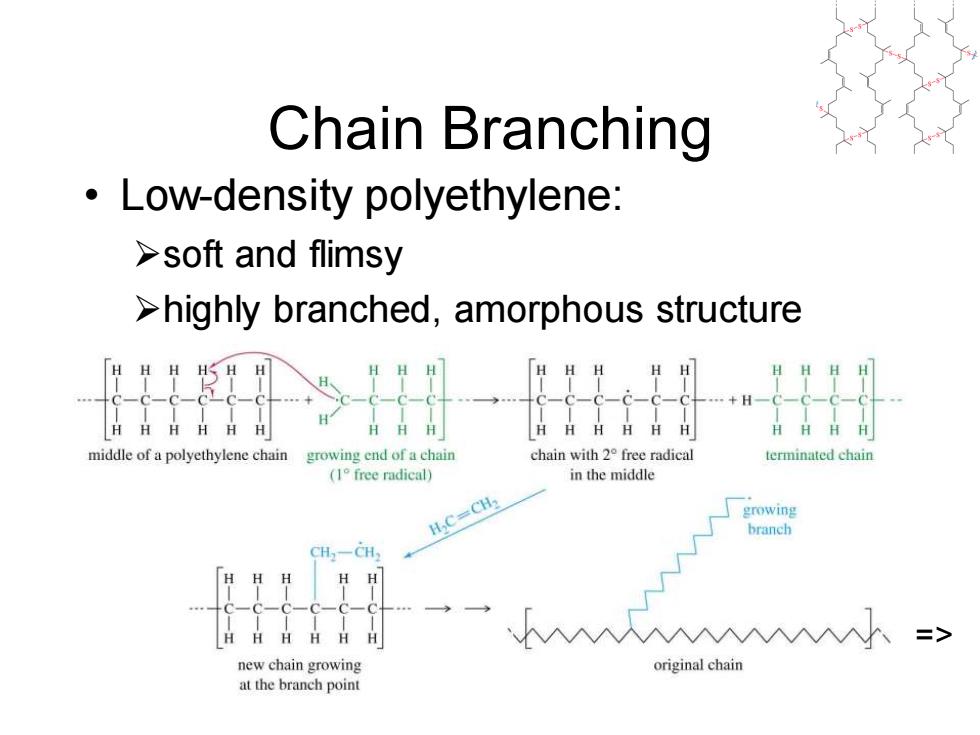
Chain Branching Low-density polyethylene: >soft and flimsy >highly branched,amorphous structure middle of a polyethylene chain growing end of a chain chain with 2 free radical terminated chain (1°free radical) in the middle H2C=CH2 growing branch CH-CH HHH H HHHHH 、=> new chain growing original chain at the branch point
Chapter 26 6 Chain Branching • Low-density polyethylene: ➢soft and flimsy ➢highly branched, amorphous structure =>
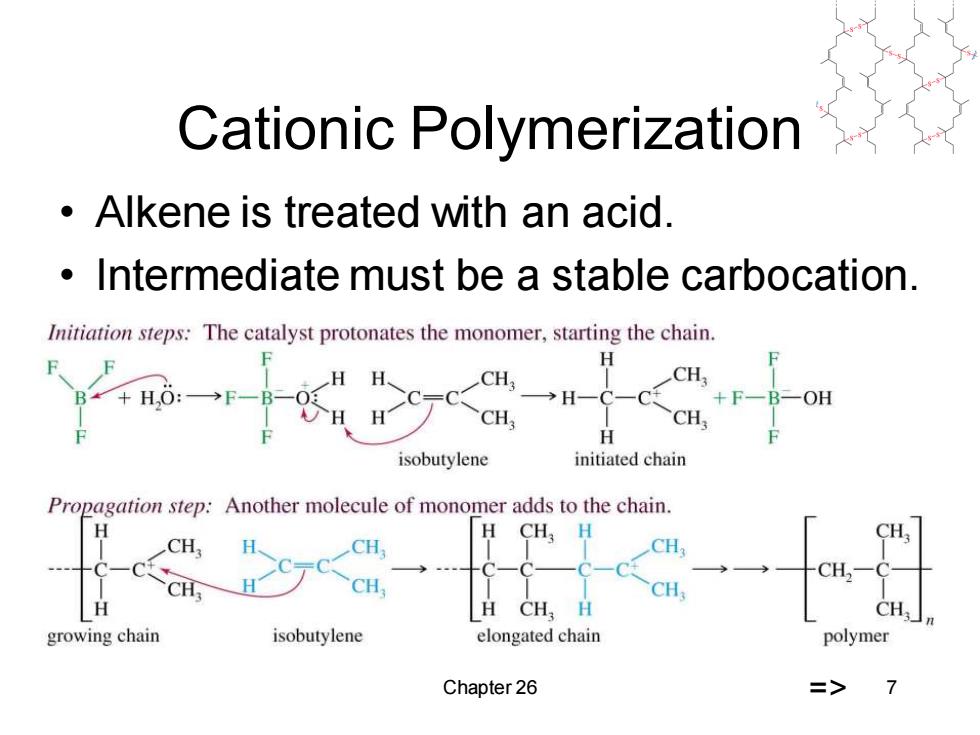
Cationic Polymerization Alkene is treated with an acid. Intermediate must be a stable carbocation. Initiation steps:The catalyst protonates the monomer,starting the chain. F H CH: B*+H,O:- CH; →H-C-C +FBOH CH, H isobutylene initiated chain Propagation step:Another molecule of monomer adds to the chain. H H CH,H CH CH CH CH,- CH CH CH H CH,H CH; growing chain isobutylene elongated chain polymer Chapter 26 => 7
Chapter 26 7 Cationic Polymerization • Alkene is treated with an acid. • Intermediate must be a stable carbocation. =>
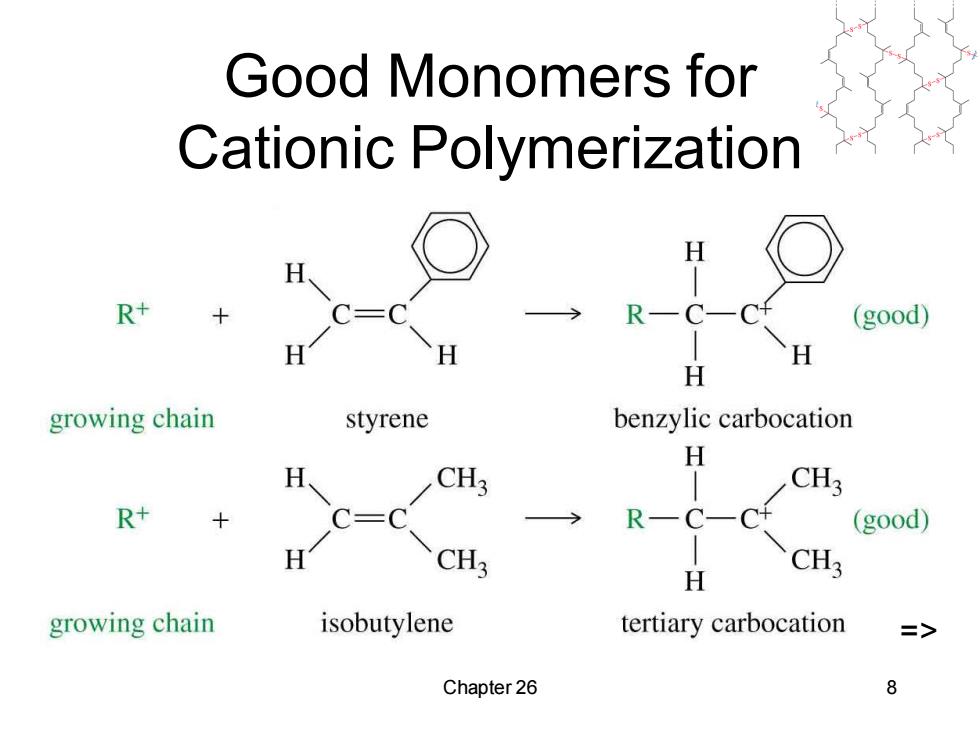
Good Monomers for Cationic Polymerization H R+ RC( (good) H H growing chain styrene benzylic carbocation H H CH3 CH3 R+ RCCH (good) CH3 H CH; growing chain isobutylene tertiary carbocation => Chapter 26 8
Chapter 26 8 Good Monomers for Cationic Polymerization =>
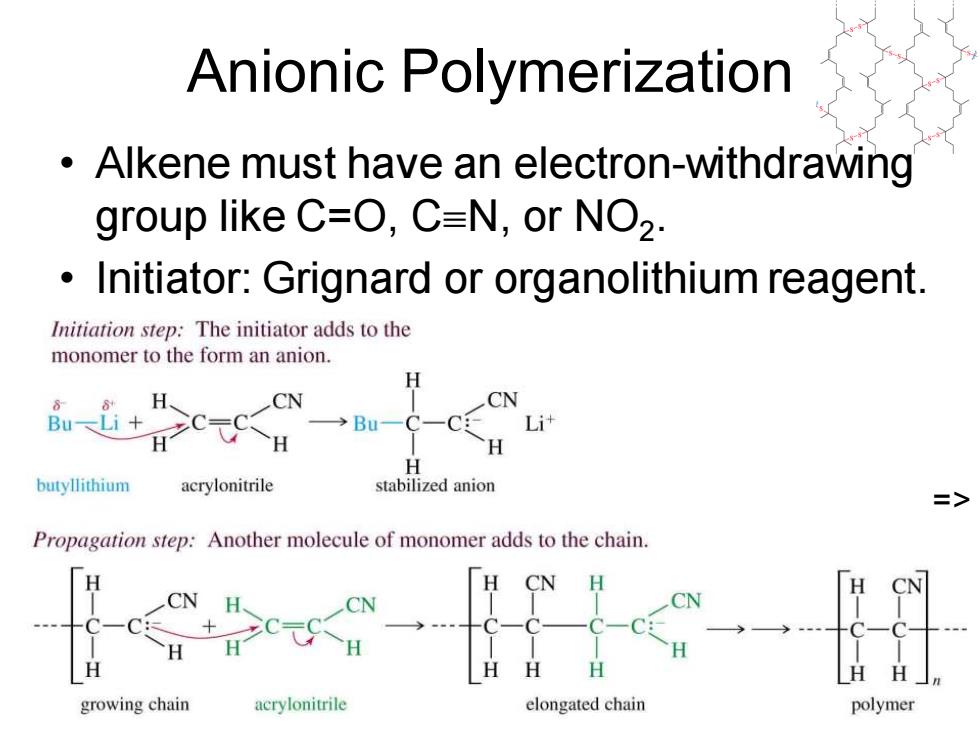
Anionic Polymerization Alkene must have an electron-withdrawing group like C=O,C=N,or NO2. Initiator:Grignard or organolithium reagent. Initiation step:The initiator adds to the monomer to the form an anion. H H CN CN Bu-Li+ →Bu Lit H H butyllithium acrylonitrile stabilized anion => Propagation step:Another molecule of monomer adds to the chain. CN H CN H H H HH」n growing chain acrylonitrile elongated chain polymer
Chapter 26 9 Anionic Polymerization • Alkene must have an electron-withdrawing group like C=O, CN, or NO2 . • Initiator: Grignard or organolithium reagent. =>
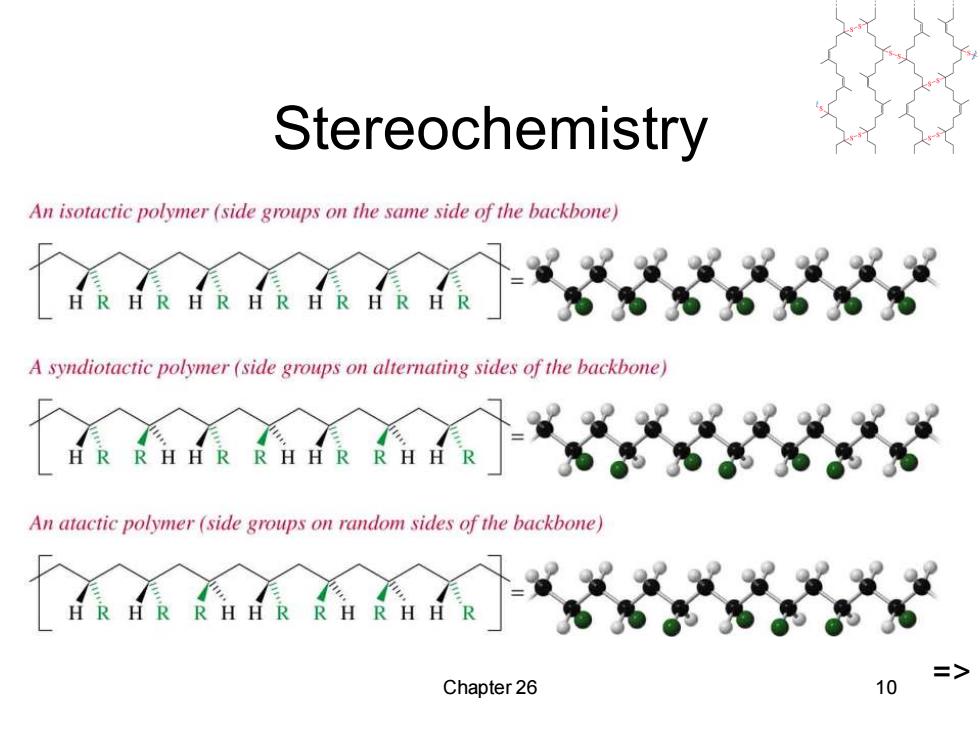
Stereochemistry An isotactic polymer (side groups on the same side of the backbone) HRHRHRHRHRHRHR A syndiotactic polymer (side groups on alternating sides of the backbone) R HH R R HH RR月方 An atactic polymer (side groups on random sides of the backbone) RH行RRHRH Chapter 26 10
Chapter 26 10 Stereochemistry =>