
Polar Covalent Bonds; Acids and Bases Based on McMurry's Organic Chemistry,6th edition,Chapter 2
Polar Covalent Bonds; Acids and Bases Based on McMurry’s Organic Chemistry, 6th edition, Chapter 2
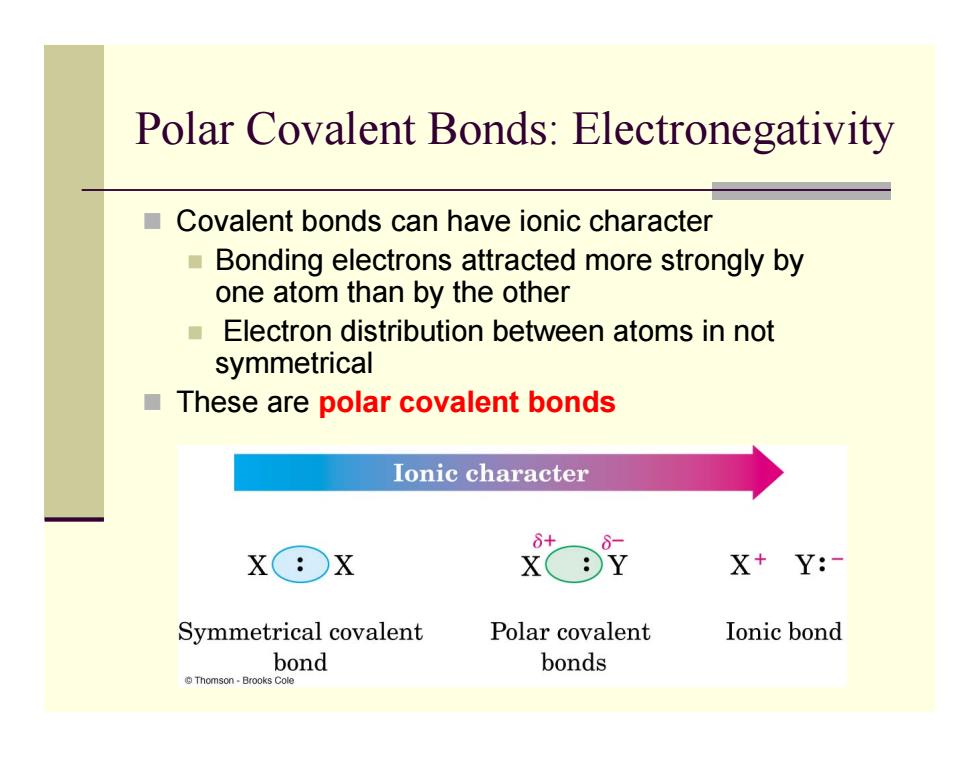
Polar Covalent Bonds:Electronegativity Covalent bonds can have ionic character Bonding electrons attracted more strongly by one atom than by the other ■ Electron distribution between atoms in not symmetrical These are polar covalent bonds Ionic character X:X X+Y:- Symmetrical covalent Polar covalent Ionic bond bond bonds Thomson-Brooks Cole
Polar Covalent Bonds: Electronegativity Covalent bonds can have ionic character Bonding electrons attracted more strongly by one atom than by the other Electron distribution between atoms in not symmetrical These are polar covalent bonds
Bond Polarity and Electronegativity ■ Electronegativity (EN):intrinsic ability of an atom to attract the shared electrons in a covalent bond Differences in EN produce bond polarity Metals on left side of periodic table attract electrons weakly,lower EN Halogens and other reactive nonmetals on right side of periodic table attract electrons strongly,higher electronegativities F is most electronegative(EN 4.0),Cs is least(EN =0.7) ■EN of C=2.5
Bond Polarity and Electronegativity Electronegativity (EN): intrinsic ability of an atom to attract the shared electrons in a covalent bond Differences in EN produce bond polarity Metals on left side of periodic table attract electrons weakly, lower EN Halogens and other reactive nonmetals on right side of periodic table attract electrons strongly, higher electronegativities F is most electronegative (EN = 4.0), Cs is least (EN = 0.7) EN of C = 2.5
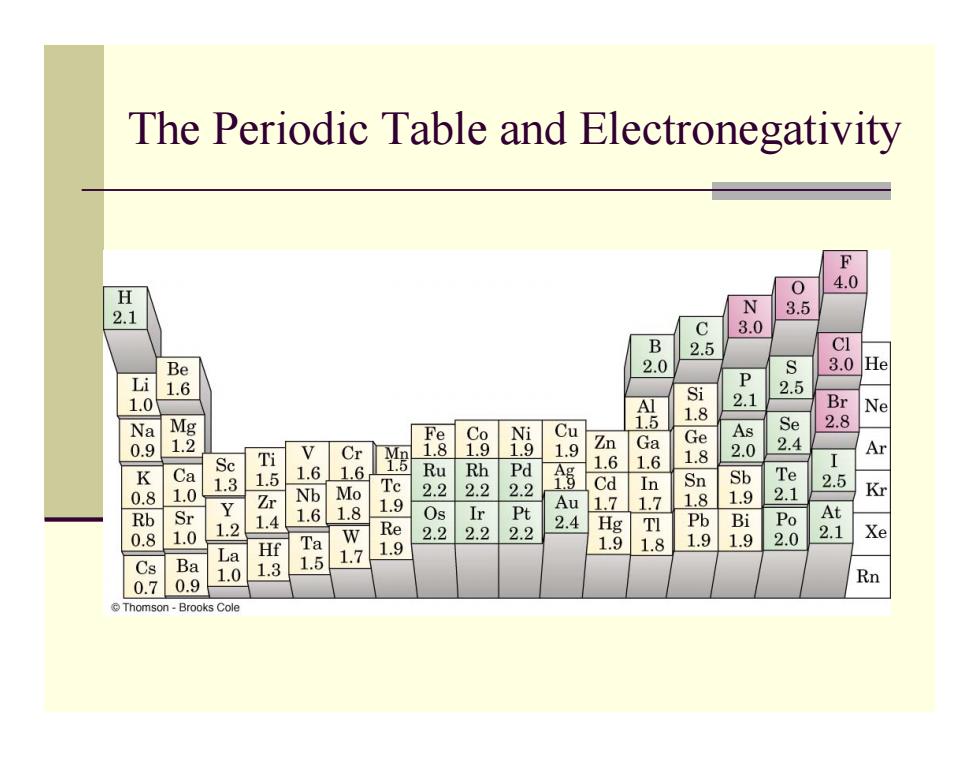
The Periodic Table and Electronegativity F 4.0 H 0 2.1 N . 3.0 B 2.5 CI Be 3.0 8 1.6 P S Mg Si 1.8 0.9 2 V Cr 遇 g a 41059 54 Br 2.8 8 Se Ti 6 I Ar 1.6 1.6 R 1.0 1.3 1.5 Te 2.2 Rh 0.8 Nb Mo 2 Cd Sn 1.9 1 2 .8 组 Kr Rb Sr 1.4 1.6 1.8 1.2 Re 2.2 2.2 2西 Hg I 的 Po At .8 1.0 Ta 1.9 1.8 .0 2.1 La H 1.9 Cs Ba . 1.5 1.7 0.70.9 .0 Rn ⊙Thomson-Brooks Cole
The Periodic Table and Electronegativity
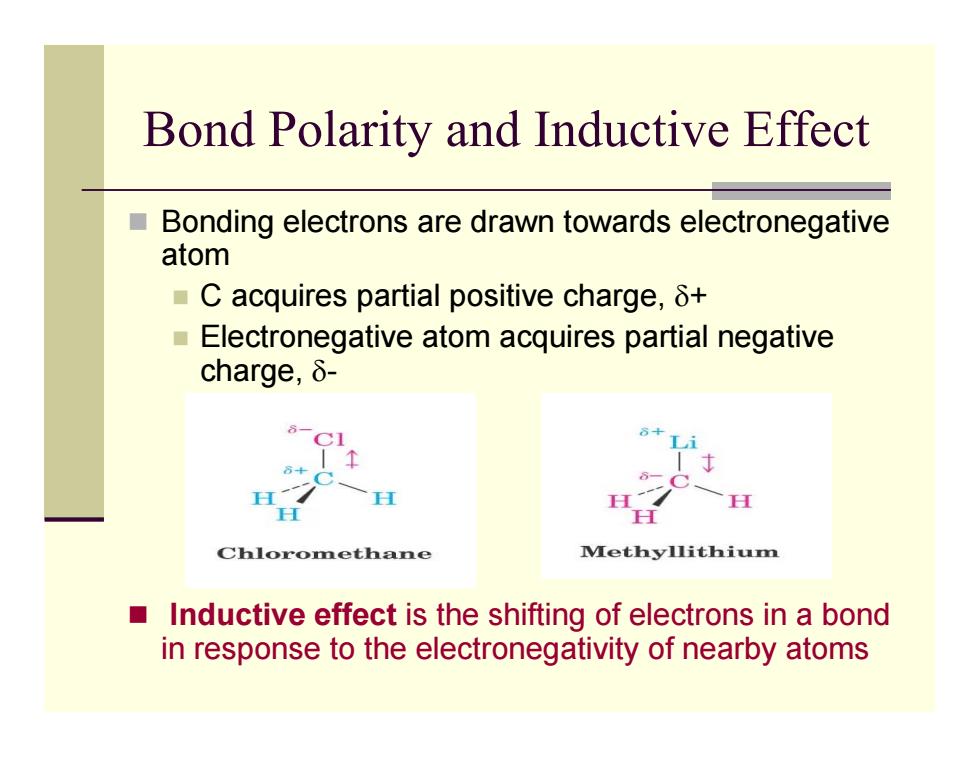
Bond Polarity and Inductive Effect ■ Bonding electrons are drawn towards electronegative atom ■C acquires partial positive charge,δt ■ Electronegative atom acquires partial negative charge,δ- H Chloromethane Methyllithium Inductive effect is the shifting of electrons in a bond in response to the electronegativity of nearby atoms
Bond Polarity and Inductive Effect Bonding electrons are drawn towards electronegative atom C acquires partial positive charge, δ + Electronegative atom acquires partial negative charge, δ - Inductive effect is the shifting of electrons in a bond in response to the electronegativity of nearby atoms
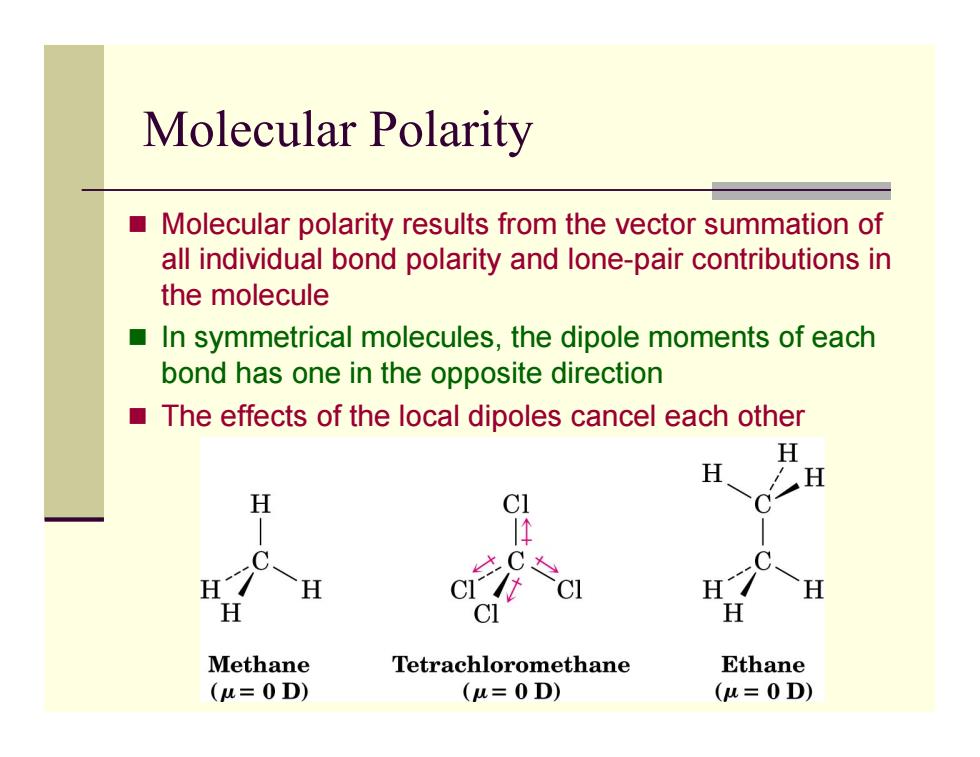
Molecular Polarity Molecular polarity results from the vector summation of all individual bond polarity and lone-pair contributions in the molecule ■ In symmetrical molecules,the dipole moments of each bond has one in the opposite direction The effects of the local dipoles cancel each other H H H H C1 必C& H H H H CI H Methane Tetrachloromethane Ethane (u=0D) (u=0D) (u=0D)
Molecular Polarity Molecular polarity results from the vector summation of all individual bond polarity and lone-pair contributions in the molecule In symmetrical molecules, the dipole moments of each bond has one in the opposite direction The effects of the local dipoles cancel each other
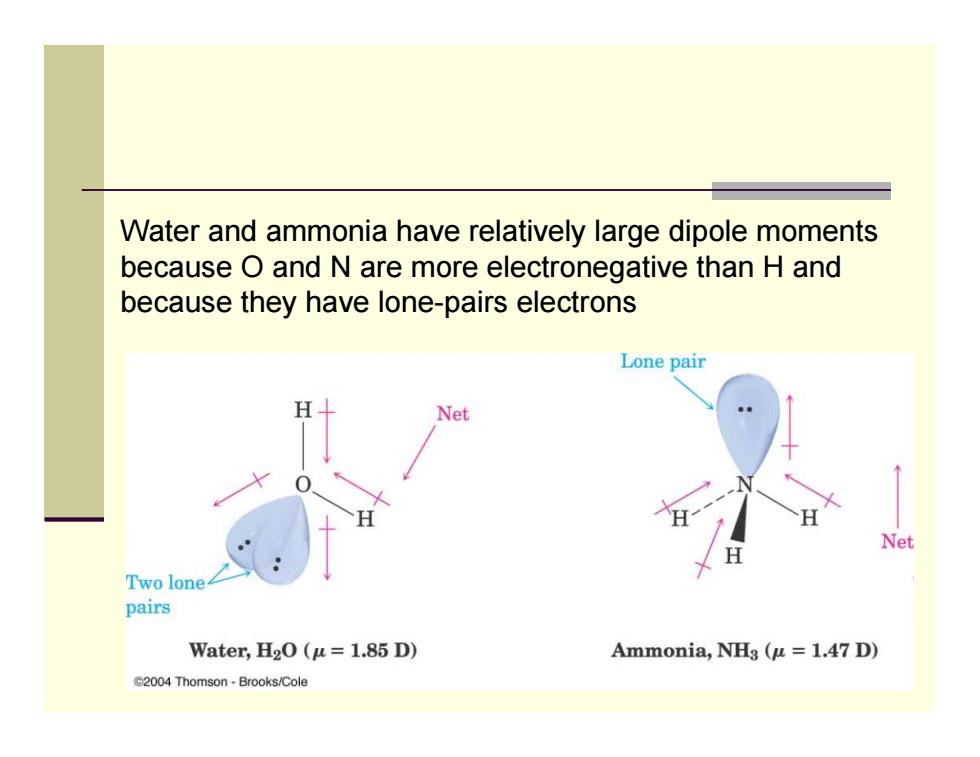
Water and ammonia have relatively large dipole moments because O and N are more electronegative than H and because they have lone-pairs electrons Lone pair Net Net Two lone pairs Water,H2O(u=1.85 D) Ammonia,NHg (u =1.47 D) G2004 Thomson-Brooks/Cole
Water and ammonia have relatively large dipole moments because O and N are more electronegative than H and because they have lone-pairs electrons
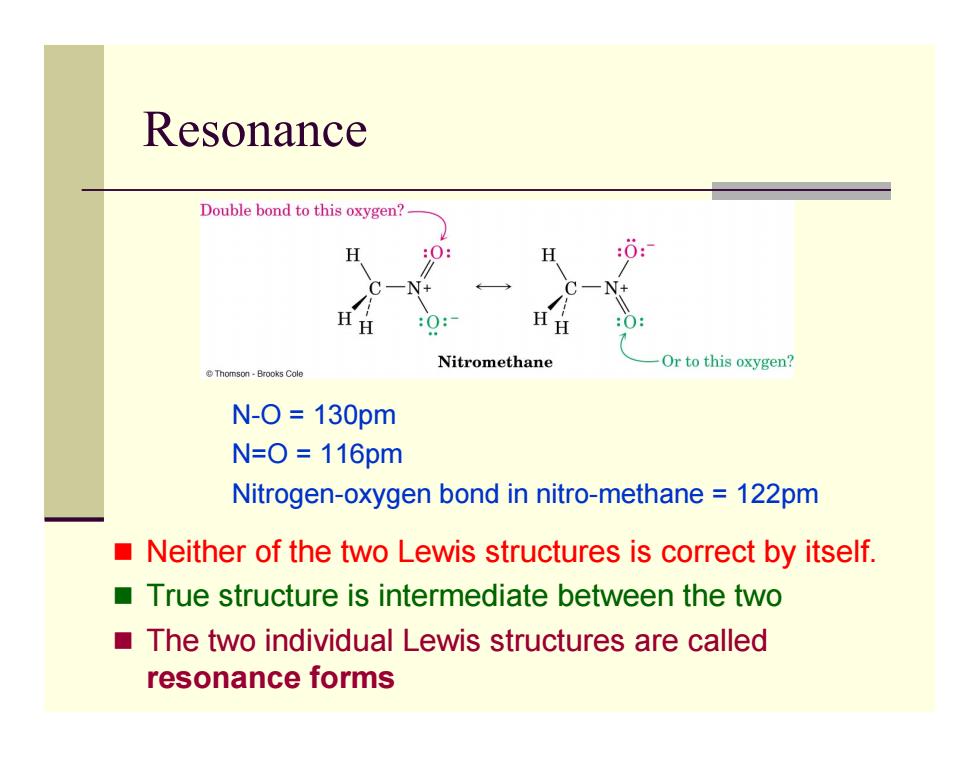
Resonance Double bond to this oxygen? H :0: Nitromethane Or to this oxygen? Thomson-Brooks Cole N-O=130pm N=0=116pm Nitrogen-oxygen bond in nitro-methane 122pm Neither of the two Lewis structures is correct by itself. True structure is intermediate between the two The two individual Lewis structures are called resonance forms
Resonance Neither of the two Lewis structures is correct by itself. True structure is intermediate between the two The two individual Lewis structures are called resonance forms N-O = 130pm N=O = 116pm Nitrogen-oxygen bond in nitro-methane = 122pm
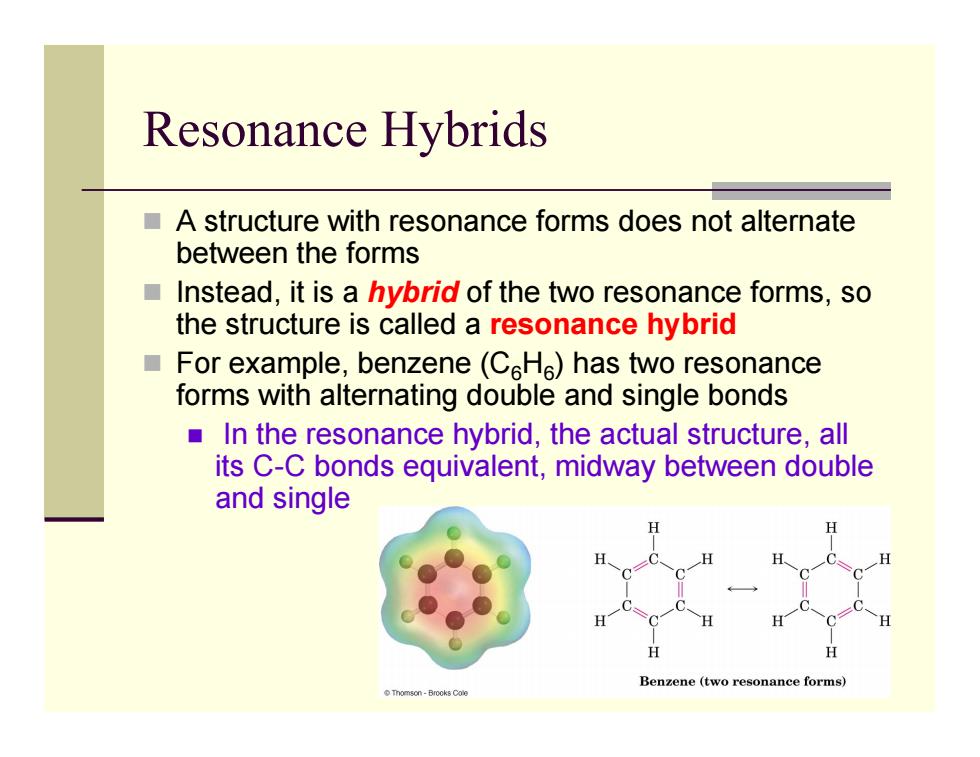
Resonance Hybrids A structure with resonance forms does not alternate between the forms Instead,it is a hybrid of the two resonance forms,so the structure is called a resonance hybrid For example,benzene(CHe)has two resonance forms with alternating double and single bonds In the resonance hybrid,the actual structure,all its C-C bonds equivalent,midway between double and single Benzene (twe nance forms Tomson-Brooks Cale
Resonance Hybrids A structure with resonance forms does not alternate between the forms Instead, it is a hybrid of the two resonance forms, so the structure is called a resonance hybrid For example, benzene (C 6 H 6) has two resonance forms with alternating double and single bonds In the resonance hybrid, the actual structure, all its C-C bonds equivalent, midway between double and single
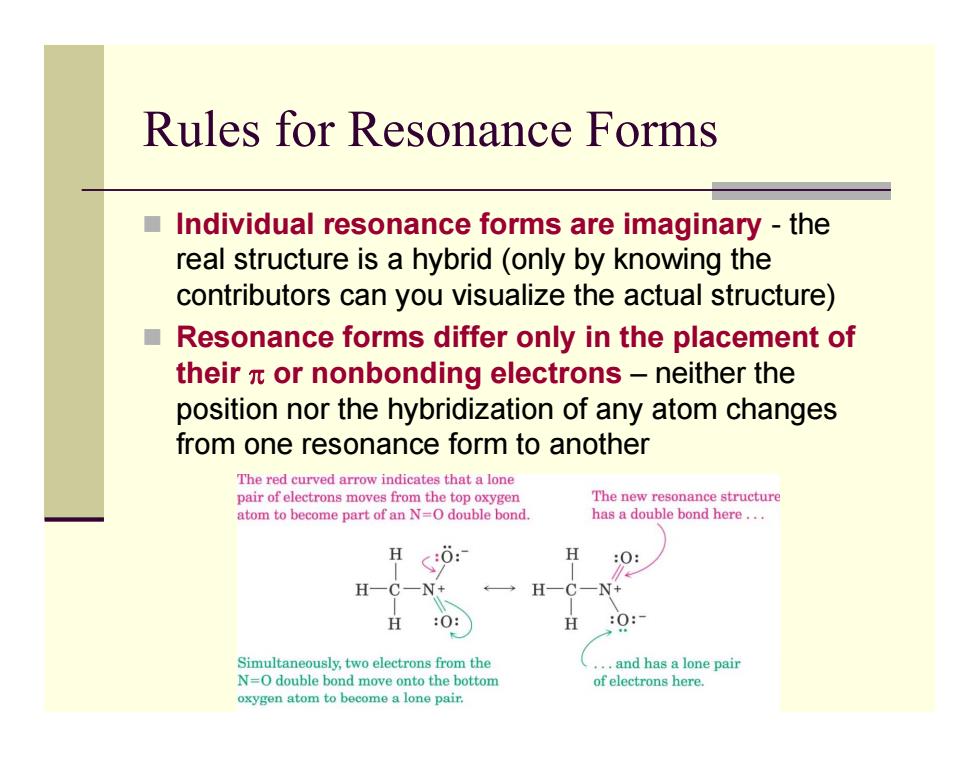
Rules for Resonance Forms Individual resonance forms are imaginary-the real structure is a hybrid (only by knowing the contributors can you visualize the actual structure) ■ Resonance forms differ only in the placement of their x or nonbonding electrons-neither the position nor the hybridization of any atom changes from one resonance form to another The red curved arrow indicates that a lone pair of electrons moves from the top oxygen The new resonance structure atom to become part of an N=O double bond. has a double bond here... H :0: H N+ H 父 O: H 0: Simultaneously,two electrons from the ..and has a lone pair N=O double bond move onto the bottom of electrons here. oxygen atom to become a lone pair
Rules for Resonance Forms Individual resonance forms are imaginary - the real structure is a hybrid (only by knowing the contributors can you visualize the actual structure) Resonance forms differ only in the placement of their π or nonbonding electrons – neither the position nor the hybridization of any atom changes from one resonance form to another