
Solutions Manual Jan William Simek California Polytechnic State University ORGANIC CHEMISTRY EIGHTH EDITION L.G.WADE,JR. PEArSON Boston Columbus Indianapolis New York San Francisco Upper Saddle River Amsterdam Cape Town Dubai London Madrid Milan Munich Paris Montreal Toronto Delhi Mexico City Sao Paulo Sydney Hong Kong Seoul Singapore Taipei Tokyo

TABLE OF CONTENTS Preface Symbols and Abbreviations Introduction and Review.... Chapter 2 Structure and Properties of Organic Molecules3 Chapter3 Structure and Stereochemistry of Alkanes... 52 Chapter 4 The Study of Chemical Reactions......... .68 Chapter 5 Stereochemistry....... 87 Chapter6 Alkyl Halides:Nucleophilic Substitution and Elimination.. 106 Chapter 7 Structare and Synthesis of Alkenes141 Chapter8 Reactions of Alkenes. .167 Chapter 9 Alkynes....... 198 Chapter10 Structure and Synthesis of Alcohols .216 Reactions of Alcohols.. 235 Chapter 12 Infrared Spectroscopy and Mass Spectrometry......................................... 264 Chapter 13 Nuclear Magnetic Resonance Spectroscopy 277 Chapter 14 Ethers,Epoxides,and Thioethers......... 306 Chapter 15 Conjugated Systems,Orbital Symmetry,and Ultraviolet Spectroscopy .326 Aromatic Compounds… .346 Chapter 17 Reactions of Aromatic Compounds 371 Chapter18 Ketones and Aldehydes. .405 Chapter 19 Amines.. 4144444444444* .439 Chapter 20 Carboxylic Acids .467 Chapter 21 Carboxylic Acid Derivatives.. 496 Chapter 22 Condensations and Alpha Substitutions of Carbonyl Compounds 531 Chapter23 Carbohydrates and Nucleic Acids. 58 Chapter24 Amino Acids,Peptides,and Proteins.... 613 Chapter 25 .641 Chapter 26 Synthetic Polymers. .657 Appendix 1:Summary of IUPAC Nomenclature of Organie Compounds 675 Appendix 2:Summary of Acidity and Basicity..... 689 Appendix 3:Sample Alkene Reaction Summary.. 701 iii

NOTES TO THE STUDENT Chapter 1 How to answer“Explain'”questions. 30 Chapter2 Wedge and dashed bond symbolism 03l Chapter 2 Functional group concept map. .40 Chapter 3 IUPAC nomenclature.. 52 Chapter3 Wedge and dashed bond symbolism reminder 55 Chapter 3 Creating practice problems in nomenclature. ..67 Chapter5 Molecular models 87 Chapter 6 Substitution and elimination concept map .140 Chapter 7 IUPAC nomenclatureⅡ. …142 Regiochemistry and major products 168 Chapter 8 Achiral reagents produce racemic products... 169 Predicting stereochemistry of alkene additions .178 Chapter 8 Deduction and inference. 193 Chapter 11 Conventions in oxidation methods. 237 Chapter 12 276 Chapter 13 Representations of benzene..... 277 Appearance of NMR spectra. 278 Chapter 16 Representations of benzene II. 346 Chapter 16 Anthropomorphizing........... .370 Chapter 18 Reminders about synthesis problems. 406 Chapter 18 Mechanism of imine formation has six steps.. 412 Chapter 18 Mechanism of acetal formation has seven steps .415 Chapter 23 Fischer projections. 581 Chapter23 Acetal and ketal concept map.... 585 Chapter 26 Polymer chain symbolism. .657 Chapter 26 Bon voyage.. 673 iv Copyright 2013 Pearson Education,Inc

PREFACE rse in organic chemistry?Here is my best advice,based on over thirty-fiv Hint #1:Do the pro rccthai humans including students.try to take seems sua he y is s you have a measured I0 above 200 and 1%o your class.do he problems.Usually your teacher (professo rcommend certain ones:try to do all those recommend If you do e your answer- -your trying and struggling with s the most aluable part of the problem.Discovery is a m jor part of that the primary goal of doing these problems is not just getting the right ans material well er gh to get right an vers to Keep course that moves as quickly as this one is rprise at the that you can study all of the material in the couple days be he but won't get a passing grade Study orga emistry like try to do some every day so that o ed Use your teacher's office hours en you h ave difficulty Many schools have tutoring centers(in which organic cher try is a popu r on absolutely the best way to cement your brain and ma and diseuss them.When ou write the up pro 11 at i ht into what this is all about. 5:When you write answers to problem,write them.Use the eold-fashioned r writing implement on paper.Keep a notebook with your work Show you impressed. Purpose of This Solutions Manual So what is the point of this So your or WhoueWwnitea its usefulnes What I can do for you is er),and at len ths to assure that what I have written is co ect,for we a shake a student's confid nce to discover that t e answe ave tried to flesh out these degree of rigor en owto solve a problem and into where the understand where students have trouble,so I have tried to anticipate your questions detail so that the concept,as well acknowledge that their teachers are human (some are uture printings What's New in This edition? Better answers!Part of my goal in this edition has been t add more explanatory material t ar conc hics!The print medium is very limited in its ability to convey three-dimensional ganic chemists for over ac Appendix 2 on Acidity has been revis a as a suggestion to student s to

Some Web Stuff http://www.masteringchemistry.com Two essential web sites providing spectra are listed on the bottom of p.276. Acknowledgments No project of this scope is ever done alone.These are team efforts.and several people who have assisted and facilitated in one fashion or another deserve my thanks. Professor L.G.Wade,Jr.,your textbook author,is a remarkable person.He has gone to extraordinary lengths to make the textbook as clear,organized,informative,and insightful as possible. He has solicited and followed many suggestions on his text,and his comments on my solutions have been perceptive and valuable.We agreed early on that our primary goal is to help the students learn a fascinating and challenging subject,and all of our efforts have been directed toward that goal.I have appreciated our collaboration. My friend and colleague,Dr.Hasan Palandoken,has reviewed the entire manuscript for accuracy and style.His extraordinary diligence,attention to detail,and chemical wisdom have made this a better manual.Hasan stands on the shoulders of previous reviewers who scoured earlier editions for errors: Dr.Kristen Meisenheimer,Jessica Gilman Erakovich.Dr.Eric Kantorowski,and Dr.Dan Mattern. Mr.Richard King of Pasadena,Texas,Editorial Adviser,has offered numerous suggestions on how to clarify murky explanations.I am grateful to them all. The people at Pearson have made this project possible.Good books would not exist without their dedication,professionalism,and experience.Among the many people who contributed are:Lee Englander,who originally connected me with this project:Jeanne Zalesky,Executive Editor in Chemistry;Jennifer Hart,Senior Project Editor in Chemistry;and Coleen MeDonald,Assistant Editor in Chemistry The entire manuscript was roduced using ChemDraw,the remarkable software for drawing chemical structures developed by CambridgeSoft Corp.,Cambridge.MA. Finally,I appreciate my friends who supported me throughout this project,most notably my wife and friend of over forty-six years,Judy Lang.The students are too numerous to list,but it is for them that all this happens. Jan William Simek.Professor Emeritus Department of Chemistry and Biochemistry Cal poly state University San Luis Obispo,CA 93407 Email:jsimek@calpoly.edu DEDICATION To my inspirational chemistry teachers: loe plaskas who made the hatter Kurt Kaufman,who baked the cake; Carl Djerassi,who put on the icing; and to m arents Ervin J.and Imilda B.Simek. who had the original concept. vi Copyright2013 Pearson Education,Inc

SYMBOLS AND ABBREVIATIONS text.Do not expect all of these to make sense to the textbook by W you now.I your study of organic chemistry) BONDS a single bond a double bond a triple bond a bond in three dimensions.coming out of the paper toward the reader a bond in three dimensions.going behind the paper away from the reader a stretched bond,in the process of forming or breaking ARROWS in a reaction,shows direction from reactants to products signifies equilibrium(not to be confused with resonance) signifies resonance(not to be confused with equilibrium) shows direction of electron movement: the arrowhe with one the arrowhead with two barb 十 shows polarity of a bond or molecule,the arrowhead signifying the more negative end of the dipole SUBSTITUENT GROUPS Me a methyl group,CH 号 an ethyl group,CH2CH3 Pr a propyl group,a three-carbon group (two possible arrangements) a butyl group,a four-carbon group(four possible arrangements) R the general abbreviation for an alkyl group(or any substituent group bonded at carbon) Ph a phenyl group,the name of a benzene ring as a substituent.represented Ar the general abbreviation for an aromatic group continued on next page vii Copyright013 Pearson Education,Ine
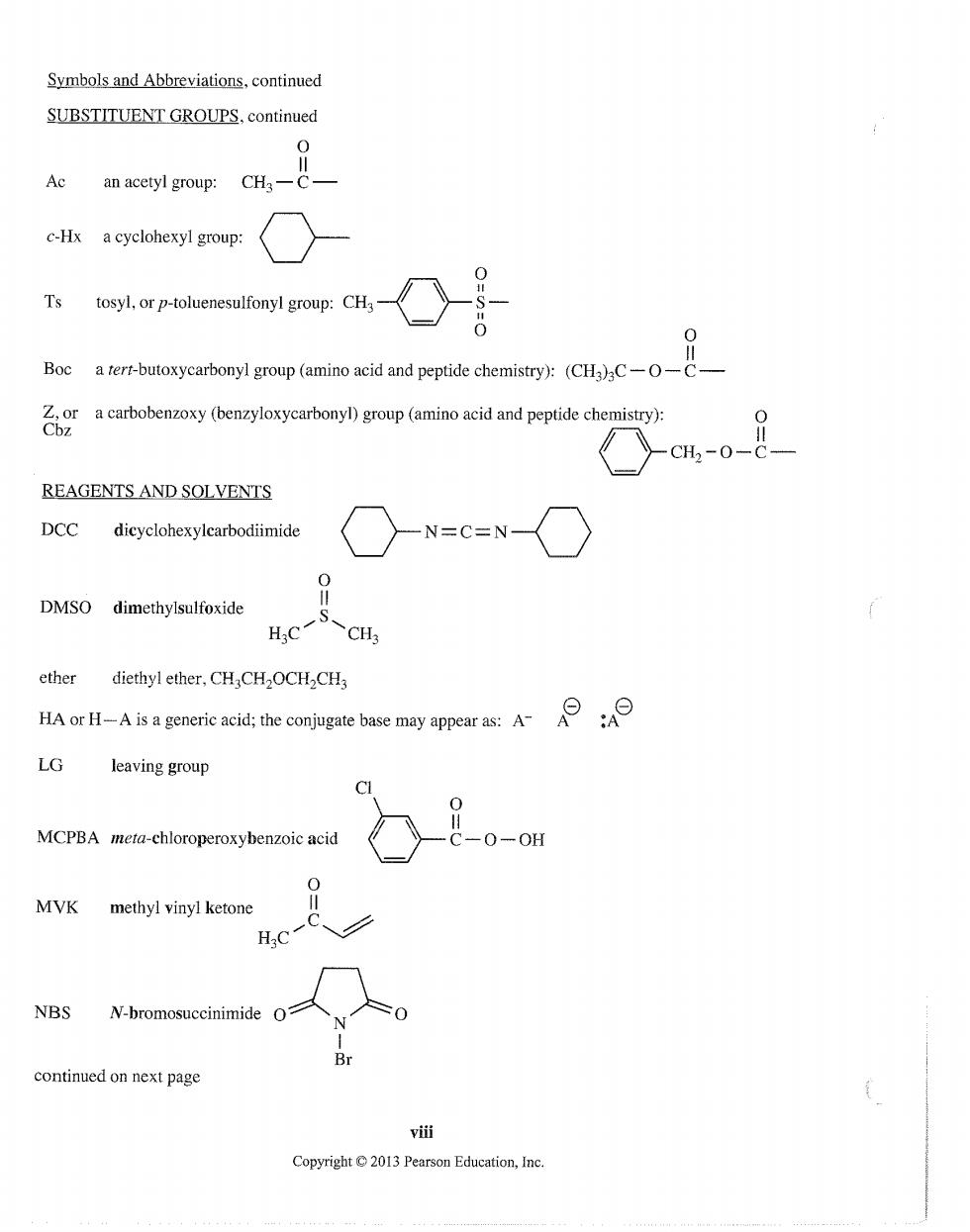
Symbols and Abbreviations,continued SUBSTITUENT GROUPS.continued an acetyl group: CHa-C :-Hx a cyclohexyl group: Ts Boc a rert-butoxycarbonyl group (amino acid and peptide chemistry):(CHa)C-- a carbobenzoxy(benzyloxycarbonyl)group(amino acid and peptide chemistry) -cH2-0-C REAGENTS AND SOLVENTS DCC -N=C=N- DMSO dimethylsulfoxide ether diethyl ether,CHCH2OCHCH generic acid:the conjugate base may appear as:A LG leaving group MCPBA meta-chloroperoxybenzoic acid -0-0 MVK methyl vinyl ketone H:C NBS W-bromosuccinimide continued on next page vi进 Copyright 2013 Pearson Education,Inc
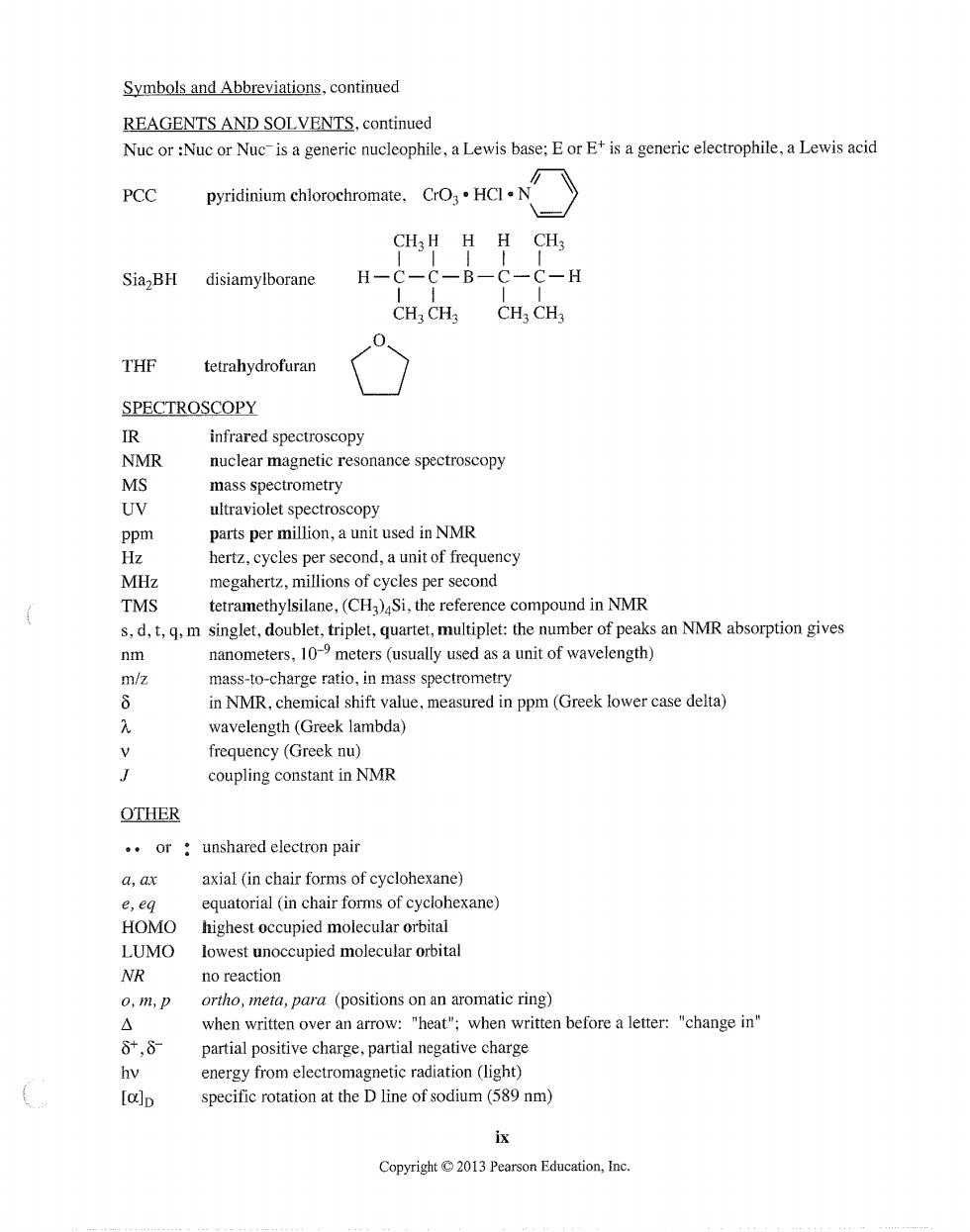
Symbols and Abbreviations,continued REAGENTS AND SOLVENTS.continued Nuc or:Nuc or Nuc-is a generic nucleophile,a Lewis base:Eor E+is a generic electrophile,a Lewis acid PCC pyridinium ehlorochromate.CrOHCIN CH3H HH CH3 SiazBH disiamylborane H -H CH:CH CH CH3 0、 THF tetrahydrofuran SPECTROSCOPY IR infrared spectroscopy NMR nuclear magnetic resonance spectroscopy MS mass spectrometry ultraviolet spectroscopy parts per million,a unit used in NMR MHz megahertz,millions of cycles per second TMS tetramethylsilane,(CH3)Si,the reference compound in NMR s.d.tm singlet,doublet,triplet,quartet,multiplet:the number of peaks an NMR absorption gives nm nanometers,10meters(usually used as a unit of wavelength) m/z mass-to-charge ratio,in mass spectrometry in NMR,chemical shift value,measured in ppm(Greek lower case delta) wavelength(Greek lambda) v frequency(Greek nu) coupling constant in NMR OTHER .or unshared electron pair a.ax axial (in chair forms of cyclohexane) e,eq equatorial (in chair forms of cyclohexane) HOMO highest occupied molecular orbital LUMO lowest unoccupied molecular orbital NR no reaction 0,m,p ortho,meta,para (positions on an aromatic ring) △ when written over an arrow:"heat";when written before a letter:"change in" 8,8 partial positive charge,partial negative charge energy from electromagnetic radiation (light) [a]p specific rotation at the D line of sodium(589 nm) ix Copyright2013 Pearson Education,I

Students:Add your own notes on symbols and abbreviations. Copyright013 Pearson Education.Inc
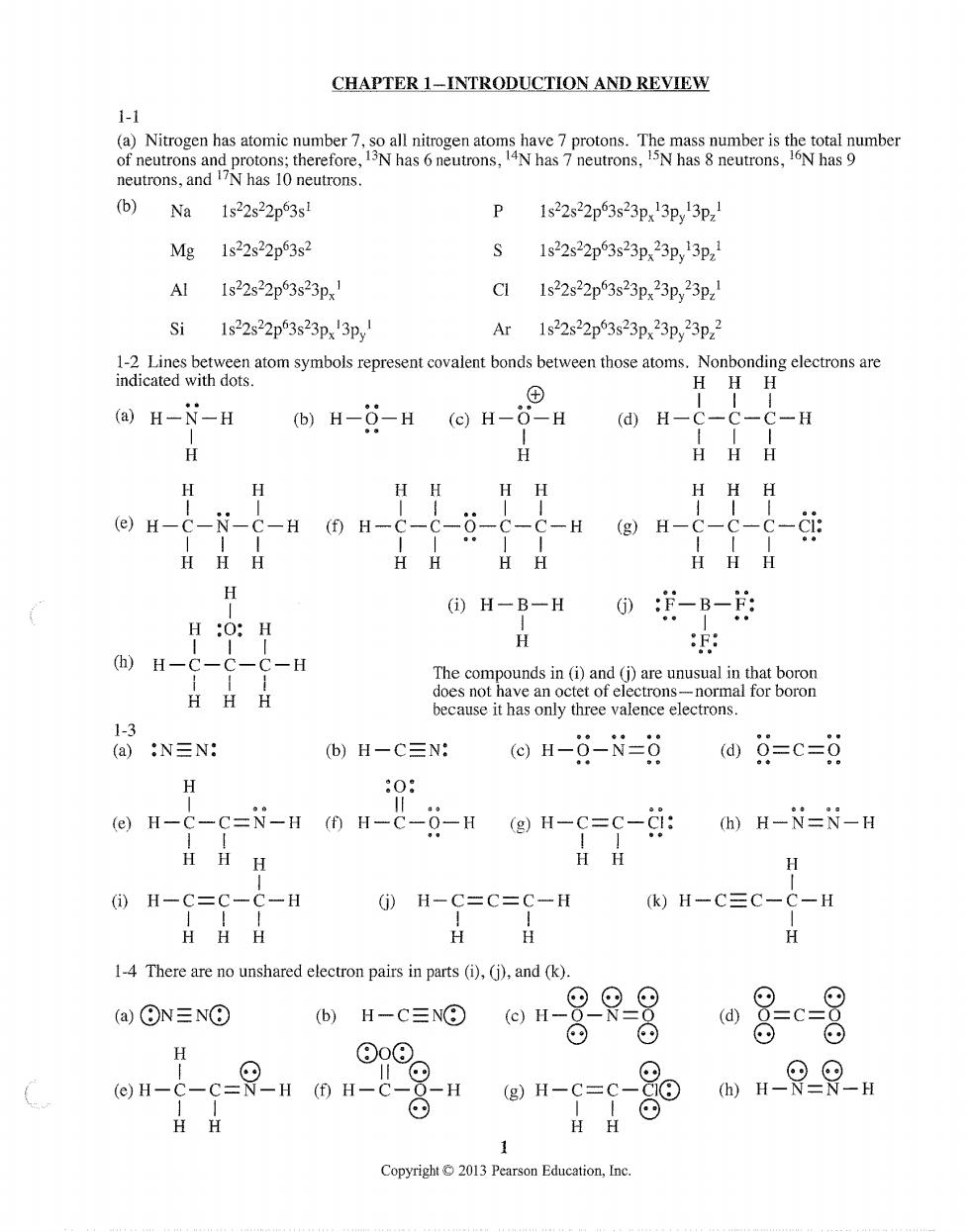
CHAPTER 1-INTRODUCTION AND REVIEW 1-1 (b)Na 1s22s22p3s! P1s22s22p3s23p,13p,13p, Mg 1s22s22p63s2 S1s22s22p63s23p,23p,13p, Al 1s22s22p3s23p C11s22s22p63s23p,23p,23pz Ar 1s2s2p3s23p,23p,23p. 品ahnmyamobprocmcowaaboahetwoatocabasnrme6kros6r HHH (a)H-N-H (b)H-0-H (e)H-6-H (d)H-C-C-C-H HHH H HH HH HHH @H-C---H0H-C-----H®H -c-ci: HHH HHHH HHH H 0H-B-H0:-B-的 H:O:H 四H----H because it has only three valence electrons. 1-3 (a):N≡N: b)H-C三N: ⊙H-9-N=日@Q=c=g H (H-c-C=-H (0 H-c-g-H (g)H-c=c-Ci:(H-N=N-H HHH HH (i)H-C=C-C-H H-C=C=C-H k)H-C三C-C-H H 1-4 There are no unshared electron pairs in parts (i),(j),and (k). (a)⊙N=NO 四-C三O因H-是只8 g (e)H- H(H-C H 11 n-6=6-80-9 HH Copyright2013 Pearson Education,Ine