
Susan McMurry Study Guide and Student Solutions Manual for McMurry's ORGANIC CHEMISTRY 7e

Contents Solutions to Problems Chapter 1 Str o 20 apt Their Stereoch 64 02 lk An Introduction Synthesis 159 Chapter 9 Stereochemistry 185 Chapter 231 Review Unit 4 Chapter 13 pvopy 26g Chapter 15 317 Chapter 16 stry of Benzene: Electrophilic Aromatic Substitution 359 Chapter 17 Is and Sulfides 437 Chapter Aldehyd s and Ketor cleophilic Addition Reactions 468 Chapter 2 CarboxyAcid Derivatives:Nucleophilic AcyI Substitution Reactions53 nyl Alpha-Substitution Reaction 578 Chapter 23 Carbonyl s and Ho de 607 eterocycles 684 Chapter 2 687 Review Unit 10 io747 Peptides,and Proteins 719 Chapter 776 Chapter 29 Thea Chemistry of Metabolic Pathways92 Orbitals and Organic Ch 821 Polymers y:Perieyelie Reactions Review 858 Appendices Functional-Group Synthesis ents in Organis Chemist 870 877 ,888 Winn n Chemistry Answers to Review-Unit Questions

Preface may think What enters your mind when you heords organic chemistrySom of "the che the e chem gh maly the study of the con mpasses many skills that are common to othe stry canI ad to improved on the task s For example lanning an organic nthesis r cquires the skills of a umust pn your moves while looing aad,and you must keep nination problems ar problems,in which many s must b ass t e in both case st be learned and then applied to the specimen or com ound under study. The proble in the text fall into two f owle ich plicated problems rec ire you to recall facts from the text and then se one or nore of the problem-solving techniques mentioned above.As each em s,nome or struc ermination-is introduced in the Here are several suggestions that may help you with problem solving: is anizcdintoch pters tha ups.As you that gre In general knowledge of functional groups for help. 2.Use molec ula ar mo It is difficult to visualize the three-dimens onal stru ture of ar 0 cate the struct ural aspe ganic che understandinsterchemist nd are indis ools fo 3.Every effort has s been m o mak c thi Soluti cle attractive,and e ncipal use f this e ok sho ould be to check answers o worked out. The Solu ions Manual should not be used as a substitute for effort:at times struggling with a problem is the only way to teach yourself A Look thr the intormation related to the history of organic chemistry. can be used for a concis review of the text material and can also serve as a reference.After every few chapter Onit has erted.In mos n the Revie textbook where a test might be give ly the place skills needed to solve problems and several important points that might need or tha been ineue the text from a slightly different w. thay.the small self-test that has ws you to te one chapter

I have tried to include es of study aids in this Solutions Manual. this boo er and quidebook to your ions Manual is the t you nee ould like to thank my husband.hn ite this pook many years uhing .Although many people encouragement during this also woul

Chapter 1-Structure and Bonding Chapter Outline I.Atomic Structure(Sections 1.1-1.3). A.Introduction to atomic structure(Section 1.1). 1.Atoms consist of a dense,positively charged nucleus surrounded by negatively charged el made up of positively charged protons and uncharged neutrons b.The nucleus contains most of the mass of the atom. Electrons move about the nucleus at a distance of about 101m. nucleus. 4.A s and neutrons a.Atoms of a given element can have different values of A. Aroms of the same element with different values of A are called isotopes. on of electrons in an atom c an be described by a wave equation The solution to a wave equation is ar rhital re sented by b.predicts the volume of space in which an electron is likely to be found. 2.There are four different kinds of orbitals (s,p,d,A). a.The s orbitals are spherical. b.2 ell-shaped 3.An atoms leaf-shaped s are organized into shell The shells differ in the numbers and kinds of orbitals they have. s have different energies. The che 1s orbital a. next in enery. Each p orbital has a region of zero density,called a node b. C.Ele ee or itals are pand pwhich have the same energy configuration of an atom is a listing of the orbitals occupied by the electrons of the atom. 2.Rule g the ground-state electron configuration of an atom: a.Ort de ergy le · b.Only two electrons can occur all are ha henhalf-filled shell have the sam spin II.Chemical Bonding Theory (Sections 1.4-1.5). hemical bonal ng theory (Section 1.4). ravalent ouper propose affinity units"-carbon is 2.Other scientists suggested that carbon can form double bonds,triple bonds and rings

2 Chapter 1 3.Van't Hoff and Le Bel proposed that the 4 atoms to which carbon forms bonds sit 4.ofa regula tetrahedron. nd pointing B.Covalent bonds 1.Atoms bond together because the resulting compound is more stable than the ind A tion of th rest noble to form ionic con c.Atoms in the middle of the periodic table share electrons by forming covalent 2.The mber of covalent bonds formed by nds electrons it has and on the number it needs to achieve an octet. on the number of 3.Covalent bonds can be represented two ways. res,bo nds are represented as pairs of dots s drawn between two 4.Valence electrons not used for bonding are called lone-pair electrons. Lone-pair elec c ons are represented as dots. ed b the overlan of two atomic orbitals each of which contains one electron.The two electrons have posite spins. 2.Each of the bo s sha by both atoms 3. overlapping orbital symmetrical and are called a bonds Bond strength is the measure of the amount of energy needed to break a bond. Bond leng is the optimum distance erween nucle n (ond length and bond strength a. n ca mms 4 bond ith le orbita orbital b. rahedrally oriented. c.Because these orbitals are unsymmetrical,they can form stronger bonds than orbitals can geometry and a bond angle of 109.5 2. re of etha ne has the same type of hybridization as occurs C bond is forn ed rlap of two sp orbitals. angles are very close to those of methane. 1R engths and I.If one carbon 2s orbital combines with two carbon 2p orbitals,three hybrid sp nd one p orbital remains oar to 3.Two different types of bonds form between two carbons. a.A o bond forms from the overlap of two sp2orbitals. b.A n bond forms by sideways overla c

Structure and Bonding 3 obiato arbon double bond a onds for f bitals of hy en The double bond of ethylene is both shorter and stronger than the C-C bond of ethane. C.sp Orbitals (Sec 1.10 eiormcaandwbpasaiewntnangcartbon2porbital,twohybnidsporbitals 2.The two sp orbitals are 180 apart,and the two p orbitals are perpendicular to them and to other 3. wo dil type s of bonds form of two orhitals o中w,u0 qaeo e se um00ys!u0eao少 ide n of four norhitale 4.Acetylene is composed of a carbon-carbon triple bond and two o bonds formed between th g two sp orbit als of carbon and the Is orbi f hydrogen 00g.10 arbon-carbon .od td byusin hybrid prbitals 2.Both the nitrogen atom in ammonia and the oxygen atom in water form sphybrid 3.The bond angles between hydrogen and the central atom is often less than 109 because the lone-pair electrons take up more room than the o bond. 4.Beca e of their positions s in the e thir typic phosphorus and sun form more valent bond IV.Molecular hita A.Molecular orbitals arise from a mathematical combination of atomic orbitals and belong to the entire molecule. r in energy than the two b.The subtractive combination is an antibonding MO and is higher in energy than orbitals. 2.A nod the two hydrogen Is atomic rs ben nu onsaenfIedehot 3.The number of MOs in a molecule is the same as the number of ato orbitals combined V.Che structu on 112) Condensed structures don't show C-H bonds and don't show the bonds between 2. CH3,CH2 and CH units. ures are still aren't usually shown c.Other atoms are shown
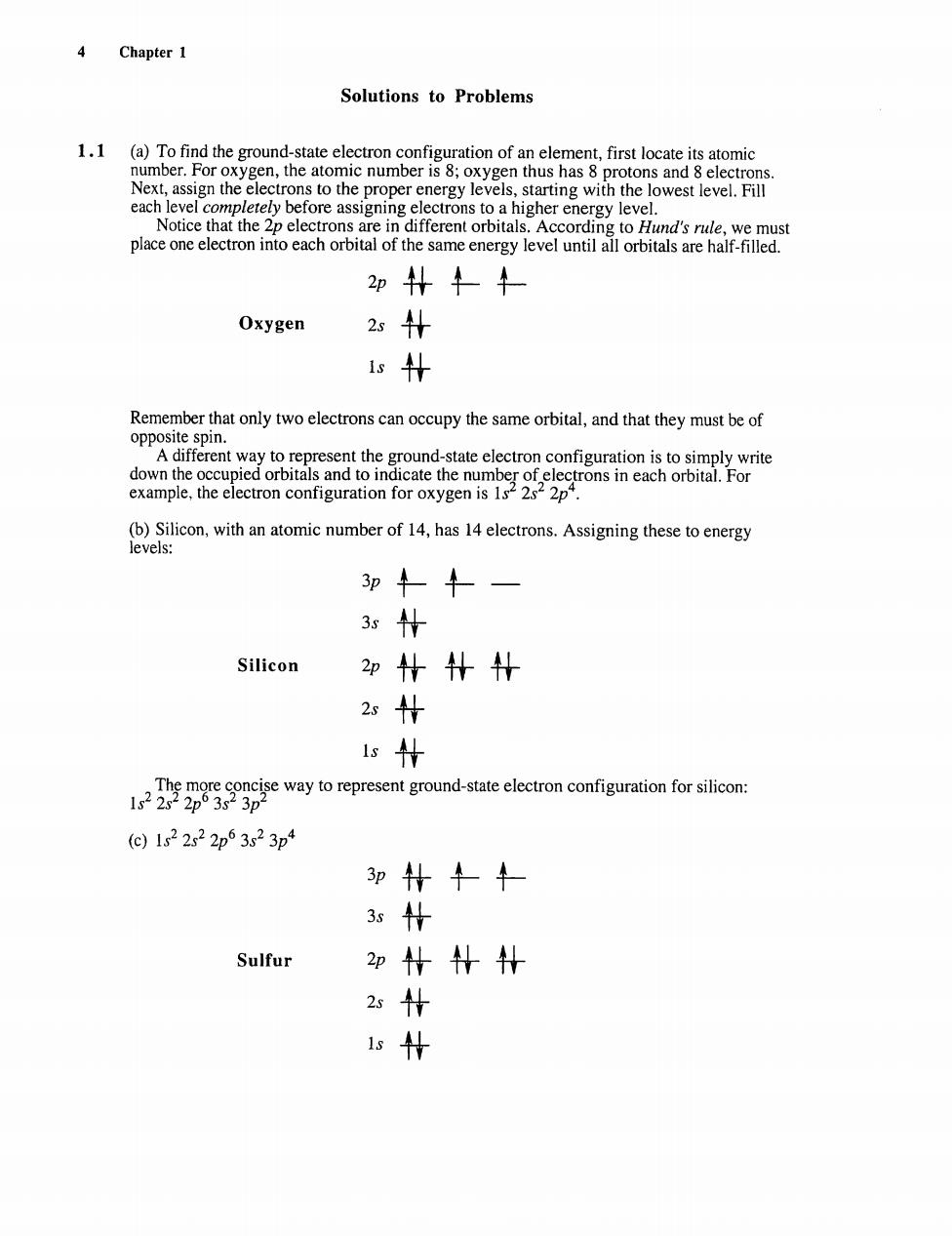
4 Chapter1 Solutions to Problems tothe proper energy levelsn with the lowest leve ore assigning electron s to a higher energy level 2p朴牛+ Oxygen 2s+ 1s+ Remember that only two electrons can occupy the same orbital,and that they must be of nd-state electror dowAecep2YoniSeaiatenageaRiHeoS example,the elctron confiuration for oxyen is 3p↓— 3s什 Silicon 2p什+H 2s Is way to represent groud-state clctron coin ori: C)122s22p53s23p4 3p什十+ 3什 Sulfur 2p什什 2什 什
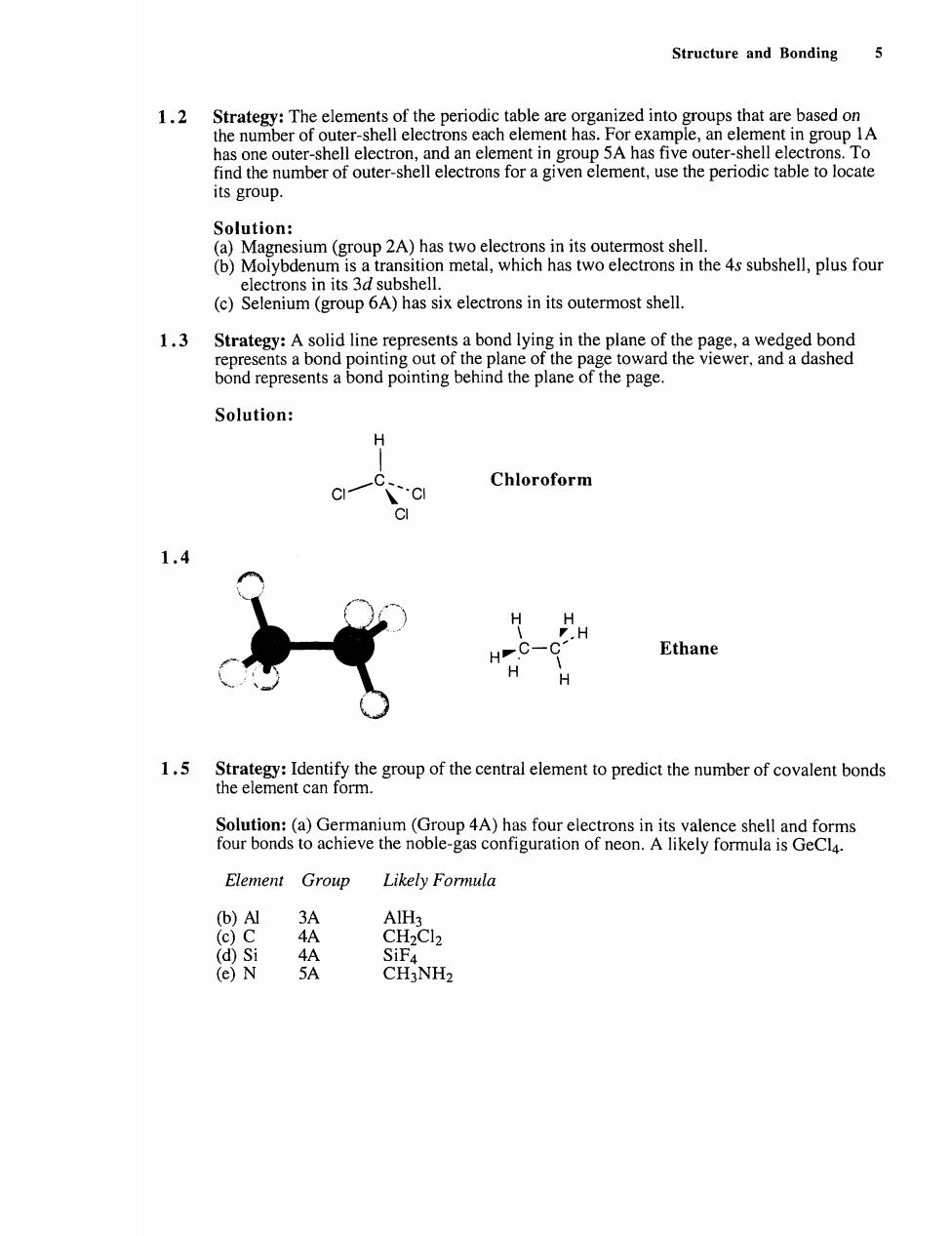
Structure and Bonding 5 1.2 he me o n h ent in group IA Solution: ons in its 3d subshell. (c)Selenium(group 6A)has six electrons in its outermost shell. 1.3 Strategy:bond A solid li ts a bond lyi bndbond pontingnd the pofpage. Solution: H CI- Chloroform 1.d H PH Ethane H 1.5 Identify the group of the central element to predict the number of covalent bonds Element Group Likely Formula (b)Al (d)Si SiF (e)N 5A CH3NH2

6 Chapter 1 1.6 Strategy:Start by drawing the electron-dot structure of the molecule. has onc valer nce elect ectrons,hydrogen a 4x1=4 H 1x1=1 :CI 7X3=21 26 total valence electrons (2)Next,use two electrons for each single bond. H CI:C:CI Solution: Molecule Electron-dot structure Line-bond structure H H (a)CHCIl3 :0:0g :c-c-: :C: 9: (b)H2S 8 valence electrons 磨 H-S: HH (c)CHaNH2 N:H 14 valence electrons H (d)CHaLi H:C:Li H--U 8 valence electrons H ons has 4 vale ce electrons Two elec hydrogens.Thus,the formula C2H is not possible