
JOHN MCMURRY THIRD EDITION Organic Chemistry with Biological Applications
42912_00_FM_i-xxiv.indd 2 1/16/14 3:00 PM
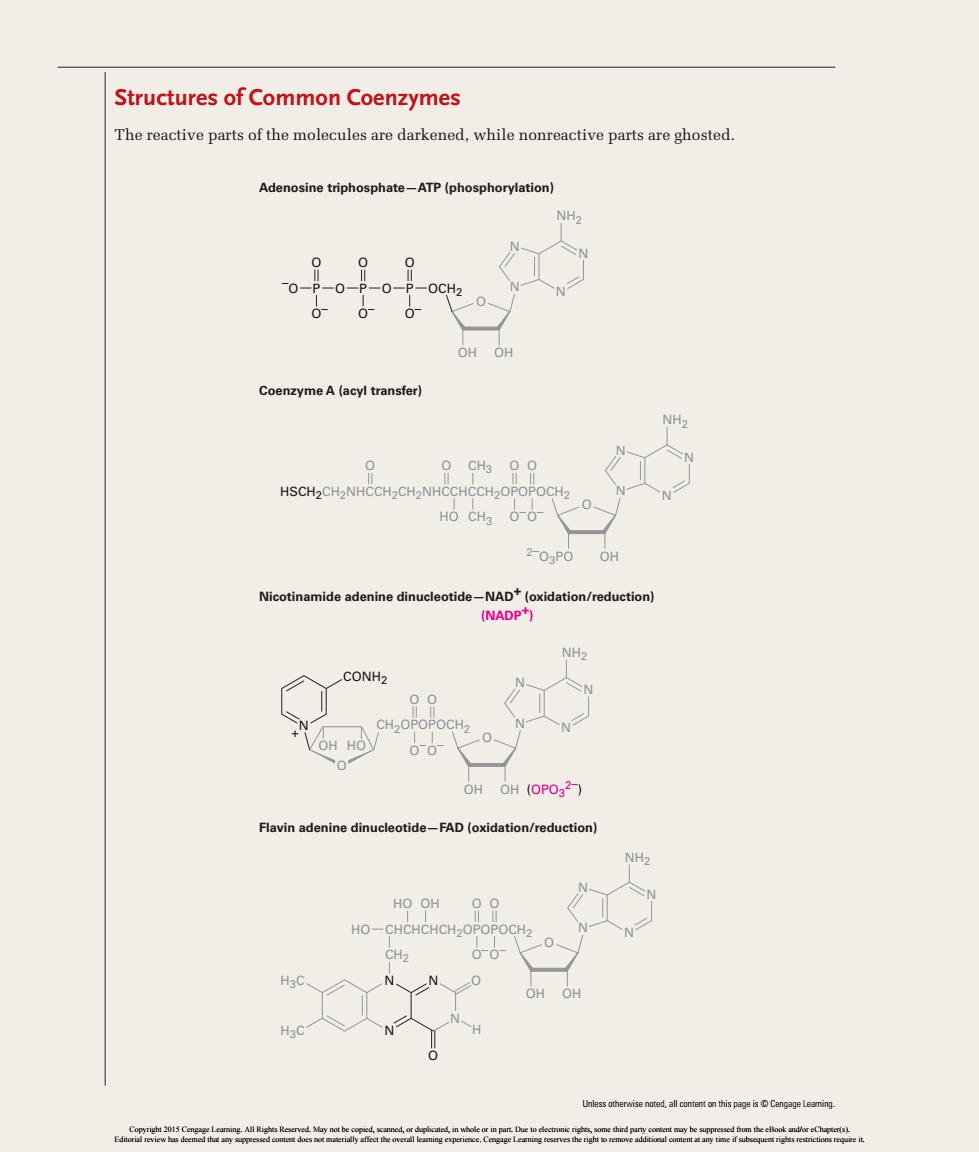
Structures of Common Coenzymes The reactive parts of the molecules are darkened,while nonreactive parts are ghosted. Adenosine triphosphate-ATP(phosphorylation) OHOH Coenzyme A(acyl transfer) 0 O CH3 HSCH2CH2NHCCH2CH2NHCCHCCH2O HO CH3 O-O- Nicotinamide adenine dinucleotide-NAD(oxidation/reduction) (NADP+) CONH2 OH OH (OPO32) Flavin adenine dinucleotide-FAD(oxidation/reduction) HO OH HO CHCHCHCH OR Ueentdllcnn
Structures of Common Coenzymes The reactive parts of the molecules are darkened, while nonreactive parts are ghosted. + CONH2 N H3C H3C N N N N O O– P –O O O O– P O OCH2 O O– P O OH Flavin adenine dinucleotide—FAD (oxidation/reduction) Nicotinamide adenine dinucleotide—NAD+ (oxidation/reduction) Coenzyme A (acyl transfer) Adenosine triphosphate—ATP (phosphorylation) OH HO CHCHCHCH2OPOPOCH2 O O– OH O O– CH2 HSCH2CH2NHCCH2CH2NHCCHCCH2OPOPOCH2 O O– O O– HO CH3 O O CH3 HO O OH OH (OPO3 2–) (NADP+) CH2OPOPOCH2 O O– O O– O O H 2–O3PO O OH O OH HO O OH OH NH2 N N N N NH2 N N N N NH2 N N N N NH2 N N N N Unless otherwise noted, all content on this page is © Cengage Learning. 42912_33_EP2-EP3.indd 2 1/10/14 1:21 PM Copyright 2015 Cengage Learning. All Rights Reserved. May not be copied, scanned, or duplicated, in whole or in part. Due to electronic rights, some third party content may be suppressed from the eBook and/or eChapter(s). Editorial review has deemed that any suppressed content does not materially affect the overall learning experience. Cengage Learning reserves the right to remove additional content at any time if subsequent rights restrictions require it
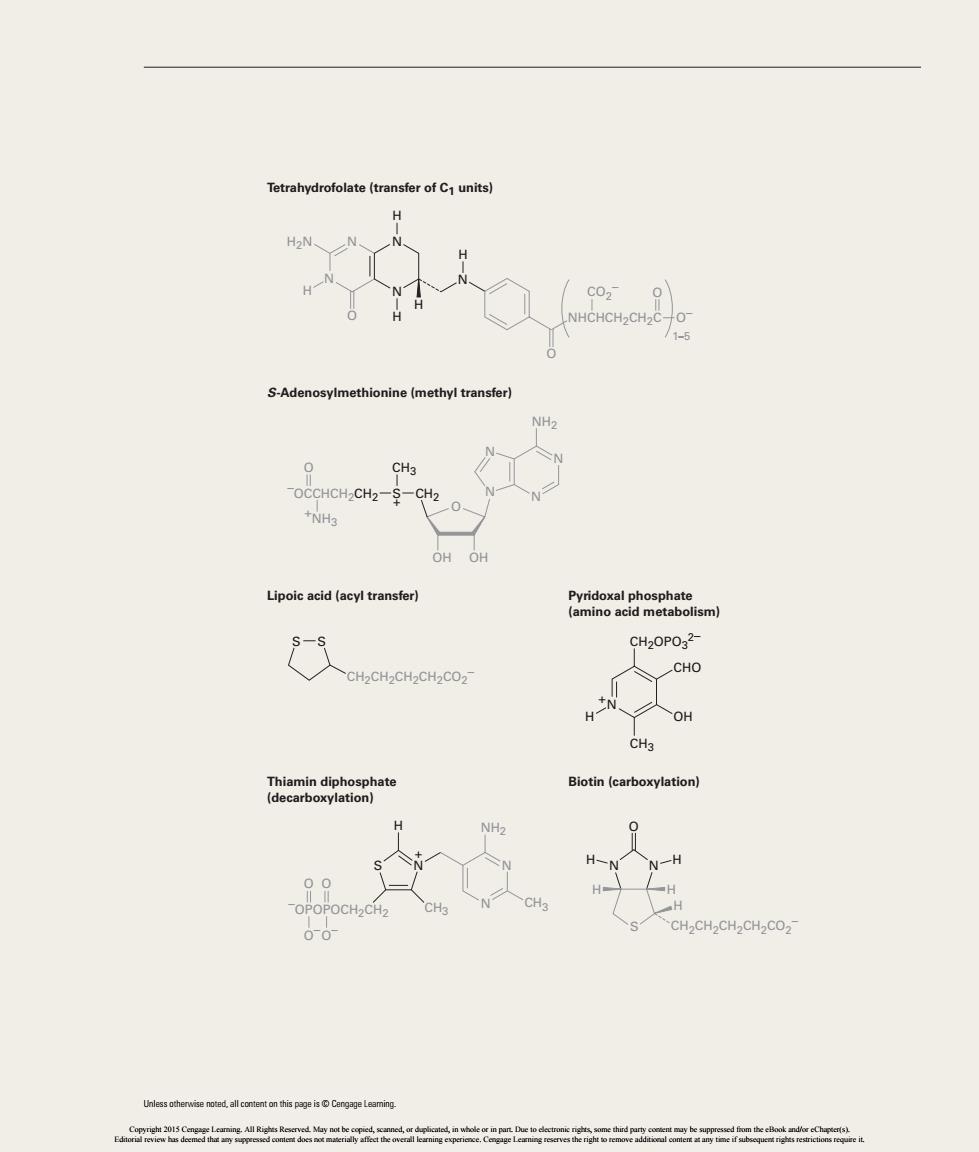
Tetrahydrofolate(transfer of C units) NHCHCH2CH2C- S-Adenosylmethionine(methyl transfer) CH3 -OCCHCH2CH2-S-CH2 +NH3 Lipoic acid(acyl transfer) CH2OPO32- CH2CH2CH2CH2CO2- CHO HnN人o CHa Biotin (carboxylation HNN一H
S-Adenosylmethionine (methyl transfer) Lipoic acid (acyl transfer) Thiamin diphosphate (decarboxylation) Pyridoxal phosphate (amino acid metabolism) Tetrahydrofolate (transfer of C1 units) CH2 –OCCHCH2CH2 CH2CH2CH2CH2CO2 – S + +NH3 O CH3 O OHOH H2N N N N N H N H H O O H H NHCHCH2CH2C O– 1–5 CO2 O – S S CH2OPO3 2– CH3 CHO OH N H + H CH3 –OPOPOCH2CH2 N + S NH2 CH3 N N O O– O O– Biotin (carboxylation) N N H H O S H H H CH2CH2CH2CH2CO2 – NH2 N N N N Unless otherwise noted, all content on this page is © Cengage Learning. 42912_33_EP2-EP3.indd 3 1/10/14 1:21 PM Copyright 2015 Cengage Learning. All Rights Reserved. May not be copied, scanned, or duplicated, in whole or in part. Due to electronic rights, some third party content may be suppressed from the eBook and/or eChapter(s). Editorial review has deemed that any suppressed content does not materially affect the overall learning experience. Cengage Learning reserves the right to remove additional content at any time if subsequent rights restrictions require it

CeingGE ko3g7t6ona 2015,2011 Cengage Learning APpl WCN:02-200-203 John McMurry Product Director:Mary Finch ALL RIGHTS RESERVED.No part of this work covered by the copyright herein Product Manager:Maureen Rosener but not limi to pn otocopying Content Developer:Sandra Kiselica obrm2tonstoaeandrenealetesecetaspemwelOneS」 Section 107or 10 of the 976United States Copyright Act.without the prior written permission of the publisher. Media Developer:Lisa Weber Marketing Manager:lulie Schuster Content Project Manager:Teresa L.Trego Cengage Lea Art Director:Maria Epes Manufacturing Planner:Judy Inouye Rights Acquisitions Specialist:Dean Dauphinais Production Service:Graphic World Inc Library of Congress Control Number:2013956751 Photo Researcher:PreMedia Global 15BN-13:978-1-285-84291-2 Text Researcher:PreMedia Global Copy Editor Graphic 15BN-10:1-285-84291-X llustrator:Graphic World In Text Designer:Parallelogram Graphics Cover Designer:Cheryl Carrington gemu4hie Cer iAmord.Groe9o2 Compositor:Graphic World Inc Cengage Learning is a leading provider of customized learning solutions with We gratefully acknowledge SDBS for providing office locations around the globe,including Singapore,the United Kingdom, data for figureson the fol data for the *404 349g.482i.and 598g(http://.aist go.jp/sdbs/,National Institute of Advanced Cengage Learing products are represented in Canada by Nelson Education,Ltd. olea more about Cengage,visit www.cengage.com Purchase any of our products at your local college store or at our preferred online store www.cengagebrain.com. Printed in the United States of America 12345671817161514
Organic Chemistry with Biological Applications, 3e John McMurry Product Director: Mary Finch Product Manager: Maureen Rosener Content Developer: Sandra Kiselica Content Coordinator: Elizabeth Woods Product Assistant: Karolina Kiwak Media Developer: Lisa Weber Marketing Manager: Julie Schuster Content Project Manager: Teresa L. Trego Art Director: Maria Epes Manufacturing Planner: Judy Inouye Rights Acquisitions Specialist: Dean Dauphinais Production Service: Graphic World Inc. Photo Researcher: PreMedia Global Text Researcher: PreMedia Global Copy Editor: Graphic World Inc. Illustrator: Graphic World Inc. Text Designer: Parallelogram Graphics Cover Designer: Cheryl Carrington Cover Image: Vickie Lewis/National Geographic Creative Compositor: Graphic World Inc. We gratefully acknowledge SDBS for providing data for figures on the following pages: 331, 335, 339, 341, 475, 476, 523, 524, 549, 672; and data for the spectra in problems on pages 349d, 349g, 482i, and 598g (http://riodb01.ibase.aist .go.jp/sdbs/, National Institute of Advanced Industrial Science and Technology, 8/26/05, 2/7/09, 2/13/09, 3/10/09). © 2015, 2011 Cengage Learning ALL RIGHTS RESERVED. No part of this work covered by the copyright herein may be reproduced, transmitted, stored, or used in any form or by any means graphic, electronic, or mechanical, including but not limited to photocopying, recording, scanning, digitizing, taping, Web distribution, information networks, or information storage and retrieval systems, except as permitted under Section 107 or 108 of the 1976 United States Copyright Act, without the prior written permission of the publisher. Library of Congress Control Number: 2013956751 ISBN-13: 978-1-285-84291-2 ISBN-10: 1-285-84291-X Cengage Learning 200 First Stamford Place, 4th Floor Stamford, CT 06902 USA Cengage Learning is a leading provider of customized learning solutions with office locations around the globe, including Singapore, the United Kingdom, Australia, Mexico, Brazil, and Japan. Locate your local office at www.cengage.com/global. Cengage Learning products are represented in Canada by Nelson Education, Ltd. To learn more about Cengage Learning Solutions, visit www.cengage.com. Purchase any of our products at your local college store or at our preferred online store www.cengagebrain.com. For product information and technology assistance, contact us at Cengage Learning Customer & Sales Support, 1-800-354-9706. For permission to use material from this text or product, submit all requests online at www.cengage.com/permissions. Further permissions questions can be e-mailed to permissionrequest@cengage.com. Printed in the United States of America 1 2 3 4 5 6 7 1 8 1 7 1 6 1 5 1 4 42912_00_FM_i-xxiv.indd 4 1/16/14 3:00 PM Copyright 2015 Cengage Learning. All Rights Reserved. May not be copied, scanned, or duplicated, in whole or in part. Due to electronic rights, some third party content may be suppressed from the eBook and/or eChapter(s). Editorial review has deemed that any suppressed content does not materially affect the overall learning experience. Cengage Learning reserves the right to remove additional content at any time if subsequent rights restrictions require it. WCN: 02-200-203

BRIEF CONTENTS 1 Structure and Bonding 1 2 Polar Covalent Bonds;Acids and Bases 28 3 Organic Compounds:Alkanes and Their Stereochemistry 9 4 Organic Compounds:Cycloalkanes and Their Stereochemistry 87 5 Stereochemistry at Tetrahedral Centers 113 6 An Overview of Organic Reactions 146 Alkenes and Alkynes 179 8 Reactions of Alkenes and Alkynes 212 9 Aromatic Compounds 265 10 Structure Determination:Mass Spectrometry,Infrared Spectroscopy,and Ultraviolet Spectroscopy 319 Structure Determination:Nuclear Magnetic Resonance Spectroscopy 350 12 Organohalides:Nucleophilic Substitutions and Eliminations 382 13 Alcohols,Phenols,and Thiols;Ethers and Sulfides 435 A Preview of Carbonyl Chemistry 483 4 Aldehydes and Ketones:Nucleophilic Addition Reactions 492 Carboxylic Acids and Nitriles 530 16 Carboxylic Acid Derivatives:Nucleophilic Acyl Substitution Reactions 555 17 Carbonyl Alpha-Substitution and Condensation Reactions 599 18 Amines and Heterocycles 644 19 Biomolecules:Amino Acids,Peptides,and Proteins 678 20 Amino Acid Metabolism 714 21 Biomolecules:Carbohydrates 738 22 Carbohydrate Metabolism 73 23 Biomolecules:Lipids and Their Metabolism 805 24 Biomolecules:Nucleic Acids and Their Metabolism 852 e25 Secondary Metabolites:An Introduction to Natural Products Chemistry 877 e26 Orbitals and Organic Chemistry:Pericyclic Reactions 905 e27 Synthetic Polymers 925
B r i e f C o n t e n t s 1 structure and Bonding 1 2 polar covalent Bonds; acids and Bases 28 3 organic compounds: alkanes and their stereochemistry 59 4 organic compounds: cycloalkanes and their stereochemistry 87 5 stereochemistry at tetrahedral centers 113 6 an overview of organic Reactions 146 7 alkenes and alkynes 179 8 Reactions of alkenes and alkynes 212 9 aromatic compounds 265 10 structure Determination: Mass spectrometry, infrared spectroscopy, and Ultraviolet spectroscopy 319 11 structure Determination: nuclear Magnetic Resonance spectroscopy 350 12 organohalides: nucleophilic substitutions and Eliminations 382 13 alcohols, phenols, and thiols; Ethers and sulfides 435 ** a preview of carbonyl chemistry 483 14 aldehydes and Ketones: nucleophilic addition Reactions 492 15 carboxylic acids and nitriles 530 16 carboxylic acid Derivatives: nucleophilic acyl substitution Reactions 555 17 carbonyl alpha-substitution and condensation Reactions 599 18 amines and heterocycles 644 19 Biomolecules: amino acids, peptides, and proteins 678 20 amino acid Metabolism 714 21 Biomolecules: carbohydrates 738 22 carbohydrate Metabolism 773 23 Biomolecules: lipids and their Metabolism 805 24 Biomolecules: nucleic acids and their Metabolism 852 To access the following online-only chapters, enter ISBN: 978-1-285-84291-2 at www.cengagebrain.com and visit this book’s companion website. e25 secondary Metabolites: an introduction to natural products chemistry 877 e26 orbitals and organic chemistry: pericyclic Reactions 905 e27 synthetic polymers 925 v 42912_00_FM_i-xxiv.indd 5 1/16/14 3:00 PM Copyright 2015 Cengage Learning. All Rights Reserved. May not be copied, scanned, or duplicated, in whole or in part. Due to electronic rights, some third party content may be suppressed from the eBook and/or eChapter(s). Editorial review has deemed that any suppressed content does not materially affect the overall learning experience. Cengage Learning reserves the right to remove additional content at any time if subsequent rights restrictions require it

DETAILED CONTENTS Structure and Bonding1 1-1 Atomic Structure:The Nucleus 3 1-2 Atomic Structure:Orbitals 4 13 Atomic Structure:Electron Configurations 6 1-4 Development of Chemical Bonding Theory 1-5 Describing Chemical Bonds:Valence Bond Theory 10 1-6 sp3 Hybrid Orbitals and the Structure of Methane 12 17 sp3 Hybrid Orbitals and the Structure of Ethane 13 1-8 sp2 Hybrid Orbitals and the Structure of Ethylene 14 1-9 sp Hybrid Orbitals and the Structure of Acetylene 16 1-10 Hybridization of Nitrogen,Oxygen,Phosphorus,and Sulfur 18 1-11 Describing Chemical Bonds:Molecular Orbital Theory 20 1-12 Drawing Chemical Structures 21 SOMETHING EXTRA Organic Foods:Risk versus Benefit 24 Polar Covalent Bonds;Acids and Bases 28 21 Polar Covalent Bonds:Electronegativity 2-2 Polar Covalent Bonds:Dipole Moments 31 2-3 Formal Charges 24 Resonance 2.5 Rules for Resonance Forms 37 2-6 Drawing Resonance Forms 27 Acids and Bases:The Bronsted-Lowry Definition 42 2-8 Acid and Base Strength 29 Predicting Acid-Base Reactions from pKa Values 46 2.10 Organic Acids and Organic Bases 47 2-11 Acids and Bases:The Lewis Definition 50 2-12 Noncovalent Interactions between Molecules 54 SOMETHING EXTRA Alkaloids:From Cocaine to Dental Anesthetics
D e t a i l e D C o n t e n t s 1 structure and Bonding | 1 1-1 atomic structure: the nucleus 3 1-2 atomic structure: orbitals 4 1-3 atomic structure: Electron configurations 6 1-4 Development of chemical Bonding theory 7 1-5 Describing chemical Bonds: Valence Bond theory 10 1-6 sp3 hybrid orbitals and the structure of Methane 12 1-7 sp3 hybrid orbitals and the structure of Ethane 13 1-8 sp2 hybrid orbitals and the structure of Ethylene 14 1-9 sp hybrid orbitals and the structure of acetylene 16 1-10 hybridization of nitrogen, oxygen, phosphorus, and sulfur 18 1-11 Describing chemical Bonds: Molecular orbital theory 20 1-12 Drawing chemical structures 21 soMetHinG eXtra organic Foods: Risk versus Benefit 24 2 Polar Covalent Bonds; acids and Bases | 28 2-1 polar covalent Bonds: Electronegativity 28 2-2 polar covalent Bonds: Dipole Moments 31 2-3 Formal charges 33 2-4 Resonance 36 2-5 Rules for Resonance Forms 37 2-6 Drawing Resonance Forms 39 2-7 acids and Bases: the Brønsted–lowry Definition 42 2-8 acid and Base strength 44 2-9 predicting acid–Base Reactions from pKa Values 46 2-10 organic acids and organic Bases 47 2-11 acids and Bases: the lewis Definition 50 2-12 noncovalent interactions between Molecules 54 soMetHinG eXtra alkaloids: From cocaine to Dental anesthetics 56 vi 42912_00_FM_i-xxiv.indd 6 1/16/14 3:00 PM Copyright 2015 Cengage Learning. All Rights Reserved. May not be copied, scanned, or duplicated, in whole or in part. Due to electronic rights, some third party content may be suppressed from the eBook and/or eChapter(s). Editorial review has deemed that any suppressed content does not materially affect the overall learning experience. Cengage Learning reserves the right to remove additional content at any time if subsequent rights restrictions require it
DETAILED CONTENTS 3 Organic Compounds: Alkanes and Their Stereochemistry 59 3.1 Functional Groups 59 2 Alkanes and Alkane Isomers 33 Alkyl Groups 9 34 Naming alkanes 3-5 Properties of Alkanes 78 3-6 Conformations of Ethane 79 3 Conformations of Other Alkanes 81 SOMETHING EXTRA Gasoline 85 Organic Compounds: Cycloalkanes and Their Stereochemistry 87 41 Naming Cycloalkanes 88 42 Cis-Trans Isomerism in Cycloalkanes 91 Stability of Cycloalkanes:Ring Strain 4-4 Conformations of Cycloalkanes 95 45 Conformations of Cyclohexane 4-6 Axial and Equatorial Bonds in Cyclohexane 4-7 Conformations of Monosubstituted Cyclohexanes 10 Conformations of Disubstituted Cyclohexanes 105 4-9 Conformations of Polycyclic Molecules 108 SoMETHING EXTRA Molecular Mechanics 11m 5 Stereochemistry at Tetrahedral Centers 13 5-1 Enantiomers and the Tetrahedral Carbon 14 5-2 The Reason for Handedness in Molecules:Chirality 115 53 Optical Activity 54 Pasteur's Discovery of Enantiomers 5-5 Sequence Rules for Specifying Configuration 名
DetaileD Contents vii 3 organic Compounds: alkanes and their stereochemistry | 59 3-1 Functional groups 59 3-2 alkanes and alkane isomers 66 3-3 alkyl groups 69 3-4 naming alkanes 72 3-5 properties of alkanes 78 3-6 conformations of Ethane 79 3-7 conformations of other alkanes 81 soMetHinG eXtra gasoline 85 4 organic Compounds: Cycloalkanes and their stereochemistry | 87 4-1 naming cycloalkanes 88 4-2 cis–trans isomerism in cycloalkanes 91 4-3 stability of cycloalkanes: Ring strain 93 4-4 conformations of cycloalkanes 95 4-5 conformations of cyclohexane 97 4-6 axial and Equatorial Bonds in cyclohexane 99 4-7 conformations of Monosubstituted cyclohexanes 102 4-8 conformations of Disubstituted cyclohexanes 105 4-9 conformations of polycyclic Molecules 108 soMetHinG eXtra Molecular Mechanics 111 5 stereochemistry at tetrahedral Centers | 113 5-1 Enantiomers and the tetrahedral carbon 114 5-2 the Reason for handedness in Molecules: chirality 115 5-3 optical activity 119 5-4 pasteur’s Discovery of Enantiomers 121 5-5 sequence Rules for specifying configuration 122 42912_00_FM_i-xxiv.indd 7 1/16/14 3:00 PM Copyright 2015 Cengage Learning. All Rights Reserved. May not be copied, scanned, or duplicated, in whole or in part. Due to electronic rights, some third party content may be suppressed from the eBook and/or eChapter(s). Editorial review has deemed that any suppressed content does not materially affect the overall learning experience. Cengage Learning reserves the right to remove additional content at any time if subsequent rights restrictions require it

DETAILED CONTENTS 5.6 Diastereomers 5.) Meso Compounds 1 5-8 Racemic Mixtures and the Resolution of Enantiomers 132 5-9 A Review of Isomerism 5-10 Chirality at Nitrogen,Phosphorus,and Sulfur 13 5-11 Prochirality 38 5-12 Chirality in Nature and Chiral Environments 14 SOMETHING EXTRA Chiral Drugs An Overview of Organic Reactions 146 6-1 Kinds of Organic Reactions 146 6-2 How Organic Reactions Occur:Mechanisms 148 63 Radical reactions 6 Polar Reactions 2 65 An Example of a Polar Reaction:Addition of H2O to Ethylene 6-6 Using Curved Arrows in Polar Reaction Mechanisms 159 6-7 Describing a Reaction:Equilibria,Rates,and Energy Changes 162 6-8 Describing a Reaction:Bond Dissociation Energies 6-9 Describing a Reaction:Energy Diagrams and Transition States 68 6-10 Describing a Reaction:Intermediates 170 6-11 A Comparison between Biological Reactions and Laboratory Reactions SOMETHING EXTRA Where Do Drugs Come From? 176 Alkenes and Alkynes 179 7-1 Calculating the Degree of Unsaturation 72 180 Naming Alkenes and Alkynes 1 7-3 Cis-Trans Isomerism in Alkenes 186 Alkene Stereochemistry and the E.Z Designation 7-5 Stability of Alkenes 189 7-6 Electrophilic Addition Reactions of Alkene 195 Writing Organic Reactions 196 77 Orientation of Electrophilic Addition:Markovnikov's Rule 91 7-8 Carbocation Structure and Stability 7-9 The Hammond Postulate 203
viii DetaileD Contents 5-6 Diastereomers 127 5-7 Meso compounds 130 5-8 Racemic Mixtures and the Resolution of Enantiomers 132 5-9 a Review of isomerism 135 5-10 chirality at nitrogen, phosphorus, and sulfur 137 5-11 prochirality 138 5-12 chirality in nature and chiral Environments 141 soMetHinG eXtra chiral Drugs 143 6 an overview of organic reactions | 146 6-1 Kinds of organic Reactions 146 6-2 how organic Reactions occur: Mechanisms 148 6-3 Radical Reactions 149 6-4 polar Reactions 152 6-5 an Example of a polar Reaction: addition of h2o to Ethylene 156 6-6 Using curved arrows in polar Reaction Mechanisms 159 6-7 Describing a Reaction: Equilibria, Rates, and Energy changes 162 6-8 Describing a Reaction: Bond Dissociation Energies 166 6-9 Describing a Reaction: Energy Diagrams and transition states 168 6-10 Describing a Reaction: intermediates 170 6-11 a comparison between Biological Reactions and laboratory Reactions 173 soMetHinG eXtra where Do Drugs come From? 176 7 alkenes and alkynes | 179 7-1 calculating the Degree of Unsaturation 180 7-2 naming alkenes and alkynes 183 7-3 cis–trans isomerism in alkenes 186 7-4 alkene stereochemistry and the E,Z Designation 188 7-5 stability of alkenes 191 7-6 Electrophilic addition Reactions of alkenes 195 writing organic Reactions 196 7-7 orientation of Electrophilic addition: Markovnikov’s Rule 197 7-8 carbocation structure and stability 201 7-9 the hammond postulate 203 42912_00_FM_i-xxiv.indd 8 1/16/14 3:00 PM Copyright 2015 Cengage Learning. All Rights Reserved. May not be copied, scanned, or duplicated, in whole or in part. Due to electronic rights, some third party content may be suppressed from the eBook and/or eChapter(s). Editorial review has deemed that any suppressed content does not materially affect the overall learning experience. Cengage Learning reserves the right to remove additional content at any time if subsequent rights restrictions require it

CONTENTS 7-10 Evidence for the Mechanism of Electrophilic Additions: Carbocation Rearrangements 206 SOMETHING EXTRA Terpenes:Naturally Occurring Alkenes 209 8 Reactions of Alkenes and Alkynes 212 8-1 Preparing Alkenes:A Preview of Elimination Reactions 213 8-2 Halogenation of Alkenes 214 8-3 Halohydrins from Alkenes 84 Hydration of Alkenes 218 8-5 Reduction of Alkenes:Hydrogenation 2 8-6 Oxidation of Alkenes:Epoxidation 27 Oxidation of Alkenes:Hydroxylation 2 8-8 Oxidation of Alkenes:Cleavage to Carbonyl Compounds 231 8.9 Addition of Carbenes to Alkenes:Cyclopropane Synthesis 233 8.10 Radical Additions to Alkenes:Chain-Growth Polymers 235 811 Biological Additions of Radicals to Alkenes 240 8.12 Conjugated Dienes 241 8-13 Reactions of Conjugated Dienes 245 8-14 The Diels-Alder Cycloaddition Reactior 8-15 Reactions of Alkynes 253 SOMETHING EXTRA Natural Rubber 258 Learning Reactions 261 9 Aromatic Compounds 265 91 Naming Aromatic Compounds 266 9 Structure and Stability of Benzene 268 Aromaticity and the Huckel 4n +2 Rule 272 94 Aromatic lons and Aromatic Heterocycles 274 95 Polycyclic Aromatic Compounds 279 9-6 Reactions of Aromatic Compounds:Electrophilic Substitution 97 Alkylation and Acylation of Aromatic Rings The Friedel-Crafts Reaction 289 9-8 Substituent Effects in Electrophilic Substitutions 295
Contents ix 7-10 Evidence for the Mechanism of Electrophilic additions: carbocation Rearrangements 206 soMetHinG eXtra terpenes: naturally occurring alkenes 209 8 reactions of alkenes and alkynes | 212 8-1 preparing alkenes: a preview of Elimination Reactions 213 8-2 halogenation of alkenes 214 8-3 halohydrins from alkenes 217 8-4 hydration of alkenes 218 8-5 Reduction of alkenes: hydrogenation 223 8-6 oxidation of alkenes: Epoxidation 227 8-7 oxidation of alkenes: hydroxylation 229 8-8 oxidation of alkenes: cleavage to carbonyl compounds 231 8-9 addition of carbenes to alkenes: cyclopropane synthesis 233 8-10 Radical additions to alkenes: chain-growth polymers 235 8-11 Biological additions of Radicals to alkenes 240 8-12 conjugated Dienes 241 8-13 Reactions of conjugated Dienes 245 8-14 the Diels–alder cycloaddition Reaction 247 8-15 Reactions of alkynes 253 soMetHinG eXtra natural Rubber 258 learning Reactions 261 9 aromatic Compounds | 265 9-1 naming aromatic compounds 266 9-2 structure and stability of Benzene 268 9-3 aromaticity and the hückel 4n 1 2 Rule 272 9-4 aromatic ions and aromatic heterocycles 274 9-5 polycyclic aromatic compounds 279 9-6 Reactions of aromatic compounds: Electrophilic substitution 281 9-7 alkylation and acylation of aromatic Rings: the Friedel–crafts Reaction 289 9-8 substituent Effects in Electrophilic substitutions 295 42912_00_FM_i-xxiv.indd 9 1/16/14 3:00 PM Copyright 2015 Cengage Learning. All Rights Reserved. May not be copied, scanned, or duplicated, in whole or in part. Due to electronic rights, some third party content may be suppressed from the eBook and/or eChapter(s). Editorial review has deemed that any suppressed content does not materially affect the overall learning experience. Cengage Learning reserves the right to remove additional content at any time if subsequent rights restrictions require it

DETAILED CONTENTS 99 Nucleophilic Aromatic Substitution 303 9-10 Oxidation and Reduction of Aromatic Compounds 306 911 An Introduction to Organic Synthesis:Polysubstituted Benzenes 308 SOMETHING EXTRA Aspirin,NSAIDs,and COX-2 Inhibitors 314 Structure Determination: Mass Spectrometry,Infrared Spectroscopy, and Ultraviolet Spectroscopy 319 10-1 Mass Spectrometry of Small Molecules:Magnetic-Sector Instruments 320 10-2 Interpreting Mass Spectra 10-3 Mass Spectrometry of Some Common Functional Groups 10-4 Mass Spectrometry in Biological Chemistry:Time-of-Flight(TOF) Instruments 328 10-5 Spectroscopy and the Electromagnetic Spectrum 329 10-6 Infrared Spectroscopy 332 10-7 Interpreting Infrared Spectra 3 10-8 Infrared Spectra of Some Common Functional Groups 37 10-9 Ultraviolet Spectroscopy 34 10-10 Interpreting Ultraviolet Spectra:The Effect of Conjugation 10-11 Conjugation,Color,and the Chemistry ofVision 346 SOMETHING EXTRA X-Ray Crystallography Structure Determination:Nuclear Magnetic Resonance Spectroscopy 350 1l-1 Nuclear Magnetic Resonance Spectroscopy 350 11-2 The Nature of NMR Absorptions 11-3 Chemical Shifts 55 11-4 13C NMR Spectroscopy:Signal Averaging and FT-NMR 3 1-5 Characteristics of13C NMR Spectroscopy 358 11-6 DEPT 13C NMR Spectroscopy 11-7 Uses of 13C NMR Spectroscopy 364 1-8 H NMR Spectroscopy and Proton Equivalence 365 119 Chemical Shifts in IH NMR Spectroscopy 368
x DetaileD Contents 9-9 nucleophilic aromatic substitution 303 9-10 oxidation and Reduction of aromatic compounds 306 9-11 an introduction to organic synthesis: polysubstituted Benzenes 308 soMetHinG eXtra aspirin, nsaiDs, and coX-2 inhibitors 314 10 structure Determination: Mass spectrometry, infrared spectroscopy, and Ultraviolet spectroscopy | 319 10-1 Mass spectrometry of small Molecules: Magnetic-sector instruments 320 10-2 interpreting Mass spectra 321 10-3 Mass spectrometry of some common Functional groups 326 10-4 Mass spectrometry in Biological chemistry: time-of-Flight (toF) instruments 328 10-5 spectroscopy and the Electromagnetic spectrum 329 10-6 infrared spectroscopy 332 10-7 interpreting infrared spectra 334 10-8 infrared spectra of some common Functional groups 337 10-9 Ultraviolet spectroscopy 342 10-10 interpreting Ultraviolet spectra: the Effect of conjugation 345 10-11 conjugation, color, and the chemistry of Vision 346 soMetHinG eXtra X-Ray crystallography 348 11 structure Determination: nuclear Magnetic resonance spectroscopy | 350 11-1 nuclear Magnetic Resonance spectroscopy 350 11-2 the nature of nMR absorptions 352 11-3 chemical shifts 355 11-4 13c nMR spectroscopy: signal averaging and Ft-nMR 357 11-5 characteristics of 13c nMR spectroscopy 358 11-6 DEpt 13c nMR spectroscopy 361 11-7 Uses of 13c nMR spectroscopy 364 11-8 1 h nMR spectroscopy and proton Equivalence 365 11-9 chemical shifts in 1 h nMR spectroscopy 368 42912_00_FM_i-xxiv.indd 10 1/16/14 3:00 PM Copyright 2015 Cengage Learning. All Rights Reserved. May not be copied, scanned, or duplicated, in whole or in part. Due to electronic rights, some third party content may be suppressed from the eBook and/or eChapter(s). Editorial review has deemed that any suppressed content does not materially affect the overall learning experience. Cengage Learning reserves the right to remove additional content at any time if subsequent rights restrictions require it