Stereochemistry Based on McMurry's Organic Chemistry,6th edition,Chapter 9
Stereochemistry Based on McMurry’s Organic Chemistry, 6th edition, Chapter 9
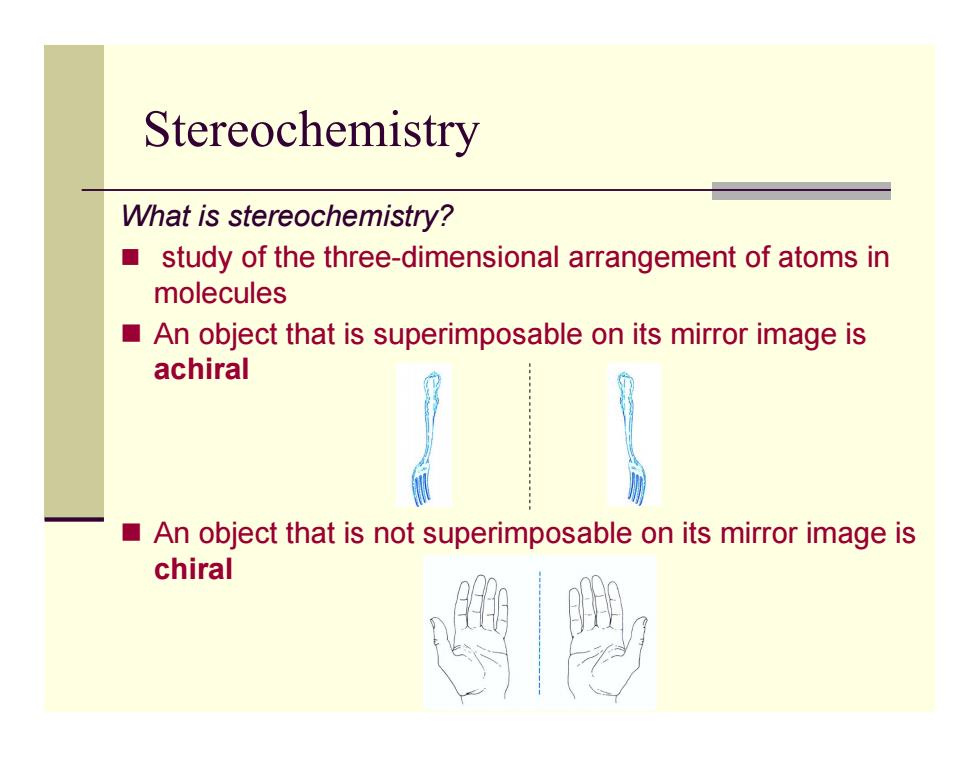
Stereochemistry What is stereochemistry? study of the three-dimensional arrangement of atoms in molecules An object that is superimposable on its mirror image is achiral An object that is not superimposable on its mirror image is chiral
Stereochemistry What is stereochemistry? study of the three-dimensional arrangement of atoms in molecules An object that is superimposable on its mirror image is achiral An object that is not superimposable on its mirror image is chiral
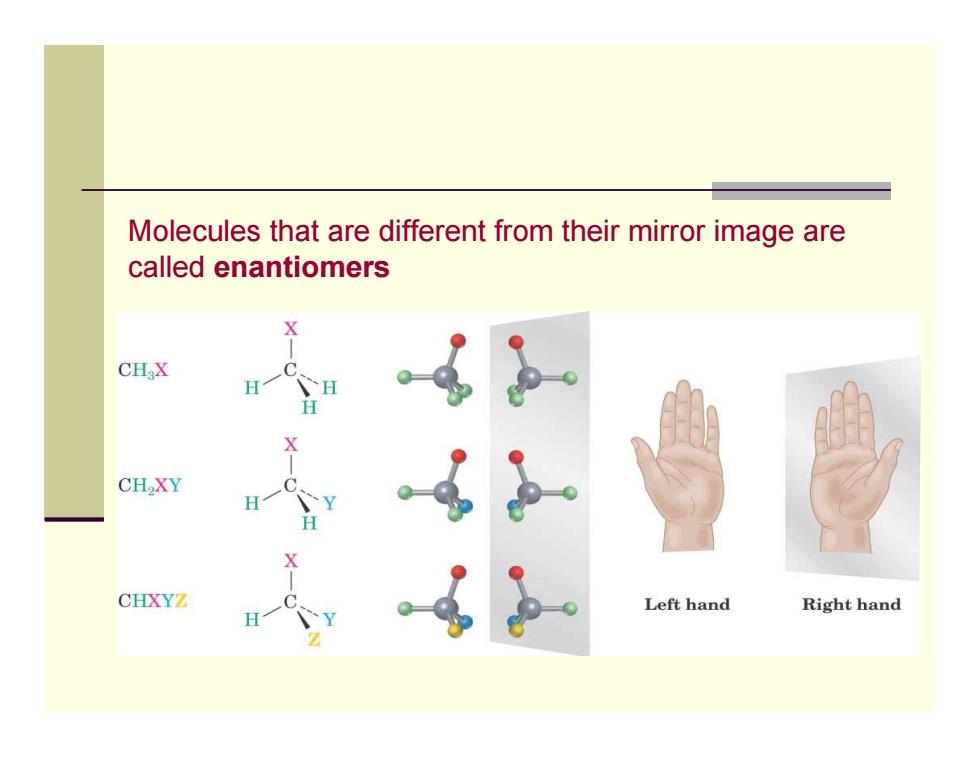
Molecules that are different from their mirror image are called enantiomers CH X CH2XY CHXYZ Left hand Right hand
Molecules that are different from their mirror image are called enantiomers
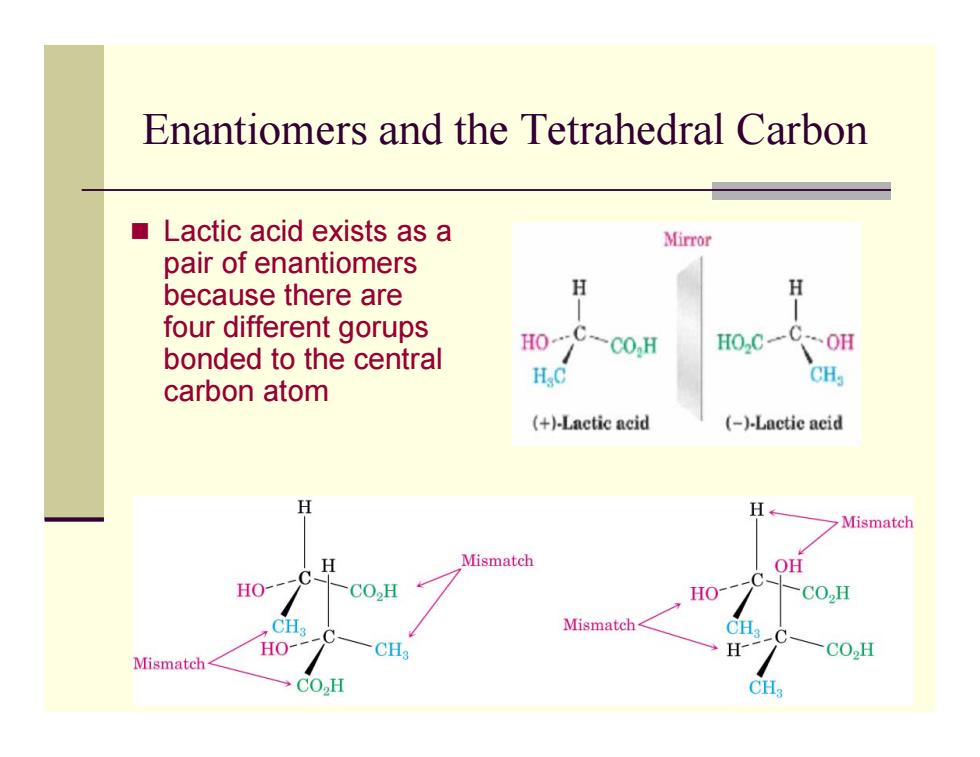
Enantiomers and the Tetrahedral Carbon Lactic acid exists as a Mirror pair of enantiomers because there are H H four different gorups HO bonded to the central C-co.H H0,C一C0H H.C CH carbon atom (+)-Lactic acid (-)-Lactic acid H H Mismatch H Mismatch OH C -C HO- CO.,H HO CO,H CH Mismatch CH. HO- Mismatch CH3 H- -CO2H CO2H CH3
Enantiomers and the Tetrahedral Carbon Lactic acid exists as a pair of enantiomers because there are four different gorups bonded to the central carbon atom

The Reason for Handedness:Chirality Achiral molecule:has a plane of symmetry and is superimposable on its mirror image A plane of symmetry is a plane that passes through a molecule in such a way that what is on one side of the plane is the exact reflection of what is on the other side NOT Symmetry symmetry plane plane CH CH H H H OH CO,H CO,H OH CH CH2CO.H CH CHCO,H Propanoic acid Lactic acid (achiral) (chiral)
The Reason for Handedness: Chirality Achiral molecule: has a plane of symmetry and is superimposable on its mirror image A plane of symmetry is a plane that passes through a molecule in such a way that what is on one side of the plane is the exact reflection of what is on the other side
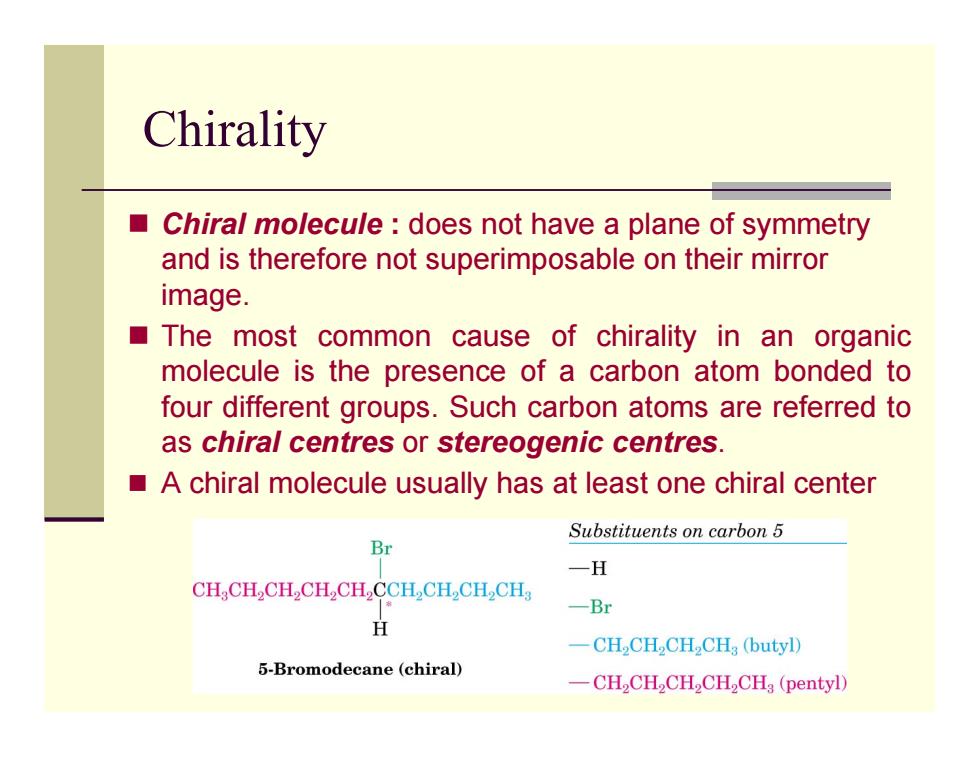
Chirality ■ Chiral molecule does not have a plane of symmetry and is therefore not superimposable on their mirror image. The most common cause of chirality in an organic molecule is the presence of a carbon atom bonded to four different groups.Such carbon atoms are referred to as chiral centres or stereogenic centres. A chiral molecule usually has at least one chiral center Substituents on carbon 5 Br 一H CHCH2CH,CH2CH,CCH,CH,CH2CH 一Br H 一CH2CHCH2CH3(butyl) 5-Bromodecane (chiral) -CH2CH2CH2CH2CHg(pentyl)
Chirality Chiral molecule : does not have a plane of symmetry and is therefore not superimposable on their mirror image. The most common cause of chirality in an organic molecule is the presence of a carbon atom bonded to four different groups. Such carbon atoms are referred to as chiral centres or stereogenic centres. A chiral molecule usually has at least one chiral center
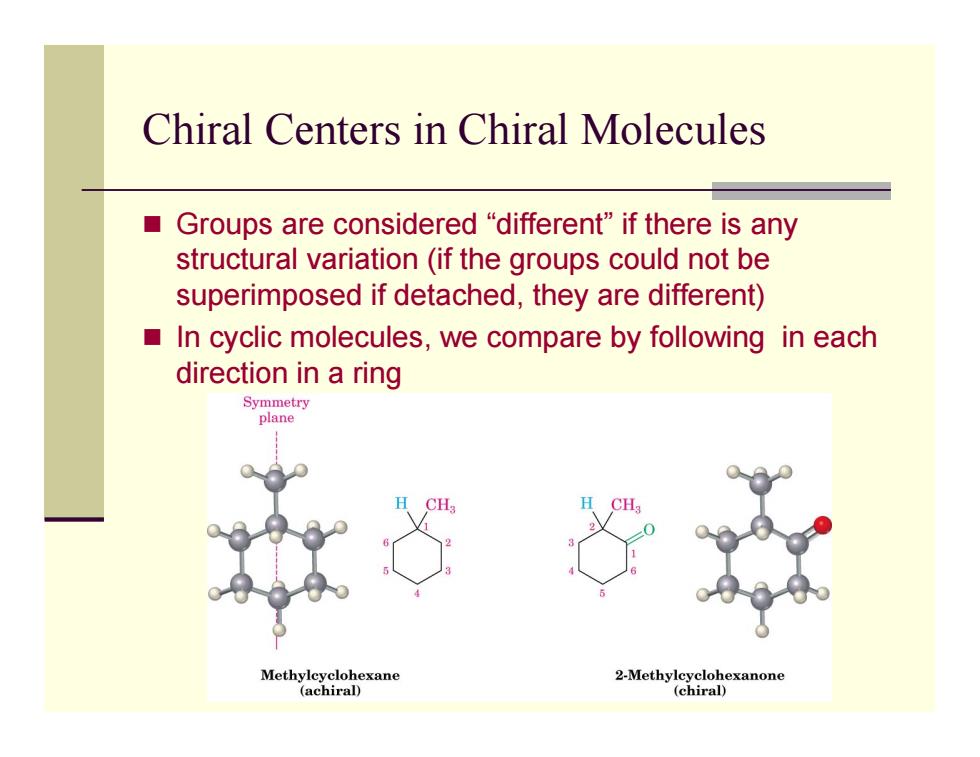
Chiral Centers in Chiral Molecules ■ Groups are considered"different"if there is any structural variation(if the groups could not be superimposed if detached,they are different) In cyclic molecules,we compare by following in each direction in a ring Symmetry plane Methylcyclohexane 2-Methylcyclohexanone (achiral) (chiral)
Chiral Centers in Chiral Molecules Groups are considered “different” if there is any structural variation (if the groups could not be superimposed if detached, they are different) In cyclic molecules, we compare by following in each direction in a ring
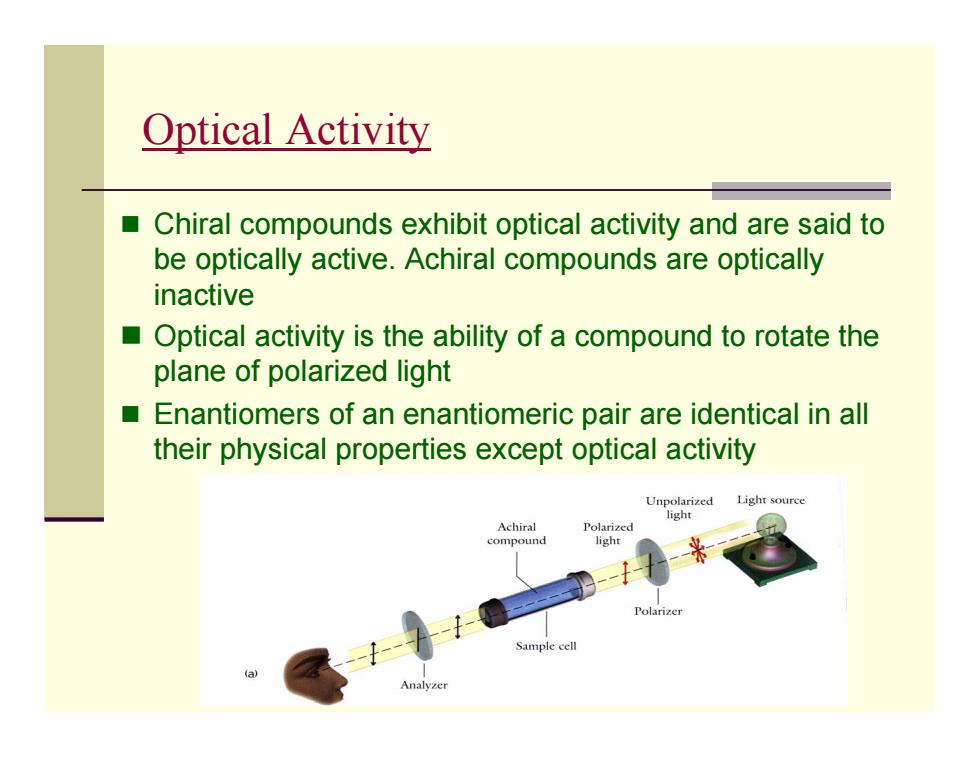
Optical activity ■ Chiral compounds exhibit optical activity and are said to be optically active.Achiral compounds are optically inactive Optical activity is the ability of a compound to rotate the plane of polarized light Enantiomers of an enantiomeric pair are identical in all their physical properties except optical activity Unpolarized Light source Achiral Polarized compound Sample cell Analyzer
Optical Activity Chiral compounds exhibit optical activity and are said to be optically active. Achiral compounds are optically inactive Optical activity is the ability of a compound to rotate the plane of polarized light Enantiomers of an enantiomeric pair are identical in all their physical properties except optical activity

Unpolarized Light source light Chiral Polarized compound light Rotated polarized ligh Sample cell The two enantiomers of a pair of enantiomers rotate the plane of polarized light by the same degree but in opposite directions One enantiomer rotates light in the clockwise direction and is called dextrorotatory (+ The other enantiomer rotates light in the anticlockwise direction and is called levorotatory(-)
The two enantiomers of a pair of enantiomers rotate the plane of polarized light by the same degree but in opposite directions One enantiomer rotates light in the clockwise direction and is called dextrorotatory (+) The other enantiomer rotates light in the anticlockwise direction and is called levorotatory (-)
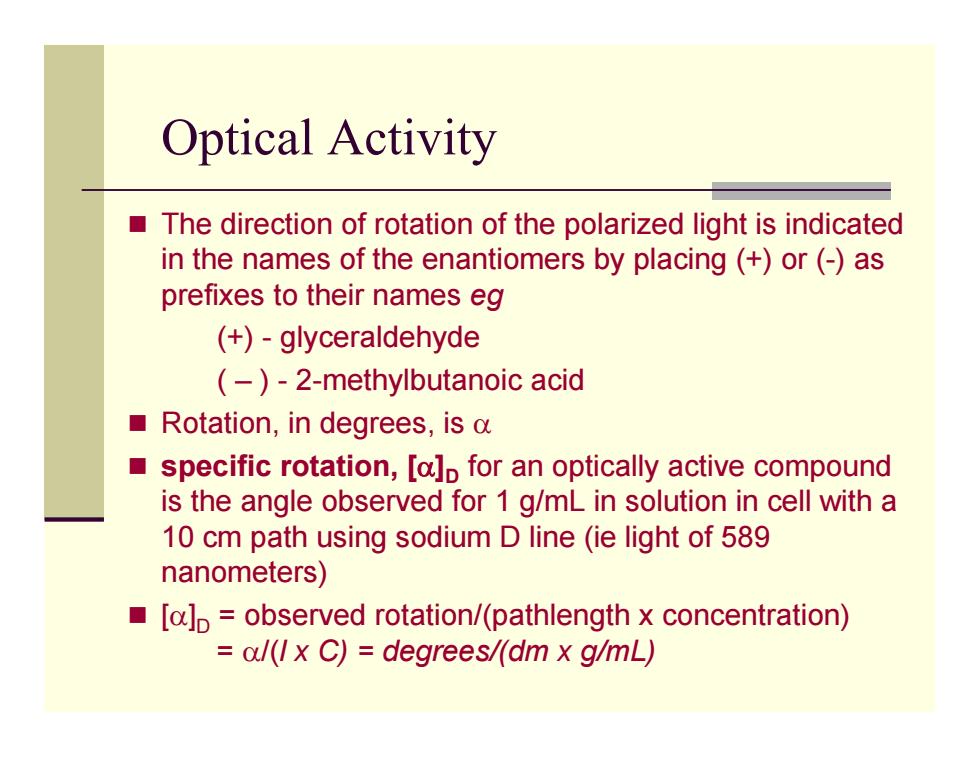
Optical Activity The direction of rotation of the polarized light is indicated in the names of the enantiomers by placing (+)or(-)as prefixes to their names eg (+)-glyceraldehyde (-)-2-methylbutanoic acid Rotation,in degrees,is a specific rotation,[a]p for an optically active compound is the angle observed for 1 g/mL in solution in cell with a 10 cm path using sodium D line (ie light of 589 nanometers) [a]p observed rotation/(pathlength x concentration) =al(Ix C)=degrees/(dm x g/mL)
Optical Activity The direction of rotation of the polarized light is indicated in the names of the enantiomers by placing (+) or (-) as prefixes to their names eg (+) - glyceraldehyde ( – ) - 2-methylbutanoic acid Rotation, in degrees, is α specific rotation, [ α ] D for an optically active compound is the angle observed for 1 g/mL in solution in cell with a 10 cm path using sodium D line (ie light of 589 nanometers) [ α ] D = observed rotation/(pathlength x concentration) = α/(l x C) = degrees/(dm x g/mL)