
Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 16 Aromatic Compounds Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2006,Prentice Hall
Chapter 16 Aromatic Compounds Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2006, Prentice Hall Organic Chemistry, 6th Edition L. G. Wade, Jr

Discovery of Benzene Isolated in 1825 by Michael Faraday who determined C:H ratio to be 1:1. Synthesized in 1834 by Eilhard Mitscherlich who determined molecular formula to be C6He. Other related compounds with low C:H ratios had a pleasant smell,so they were classified as aromatic. Chapter 16
Chapter 16 2 Discovery of Benzene • Isolated in 1825 by Michael Faraday who determined C:H ratio to be 1:1. • Synthesized in 1834 by Eilhard Mitscherlich who determined molecular formula to be C6H6 . • Other related compounds with low C:H ratios had a pleasant smell, so they were classified as aromatic. =>
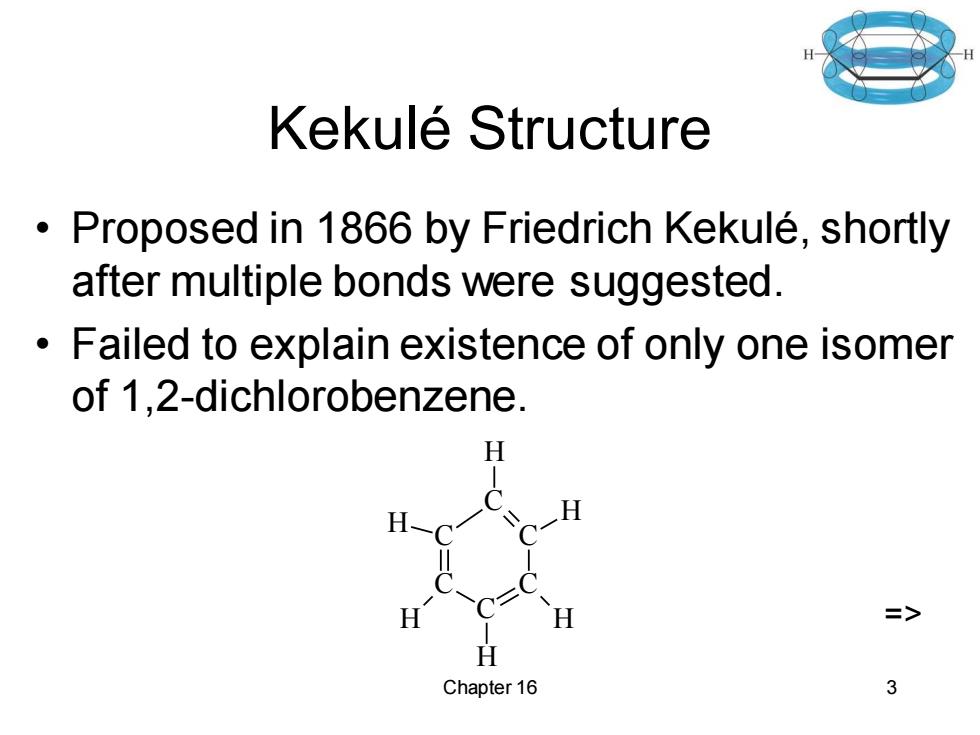
Kekule Structure Proposed in 1866 by Friedrich Kekule,shortly after multiple bonds were suggested. Failed to explain existence of only one isomer of 1,2-dichlorobenzene. H => Chapter 16 3
Chapter 16 3 Kekulé Structure • Proposed in 1866 by Friedrich Kekulé, shortly after multiple bonds were suggested. • Failed to explain existence of only one isomer of 1,2-dichlorobenzene. C C C C C C H H H H H H =>
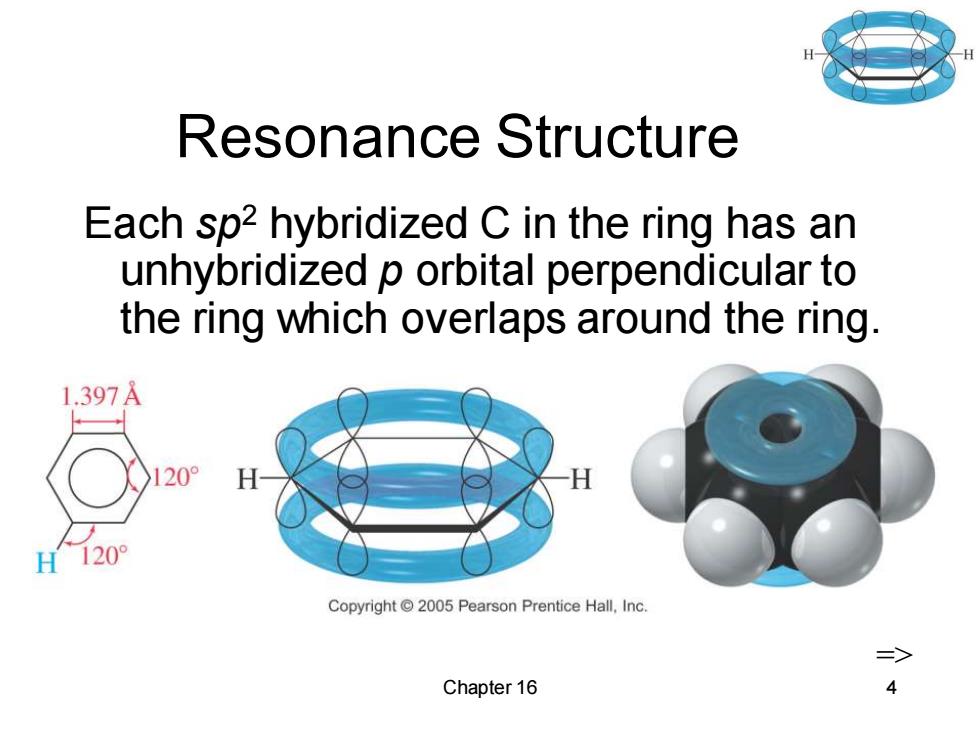
Resonance Structure Each sp2 hybridized C in the ring has an unhybridized p orbital perpendicular to the ring which overlaps around the ring. 1.397A 20 H120° Copyright2005 Pearson Prentice Hall,Inc. Chapter 16
Chapter 16 4 Resonance Structure Each sp2 hybridized C in the ring has an unhybridized p orbital perpendicular to the ring which overlaps around the ring. =>
Unusual Reactions ·Alkene+KMnO4→diol(addition) Benzene KMnO>no reaction. ·Alkene+Brz/CCl4→dibromide(addition) Benzene Br2/CCla>no reaction. With FeCla catalyst,Br2 reacts with benzene to form bromobenzene HBr (substitution!).Double bonds remain. > Chapter 16 5
Chapter 16 5 Unusual Reactions • Alkene + KMnO4 → diol (addition) Benzene + KMnO4 → no reaction. • Alkene + Br2 /CCl4 → dibromide (addition) Benzene + Br2 /CCl4 → no reaction. • With FeCl3 catalyst, Br2 reacts with benzene to form bromobenzene + HBr (substitution!). Double bonds remain. =>
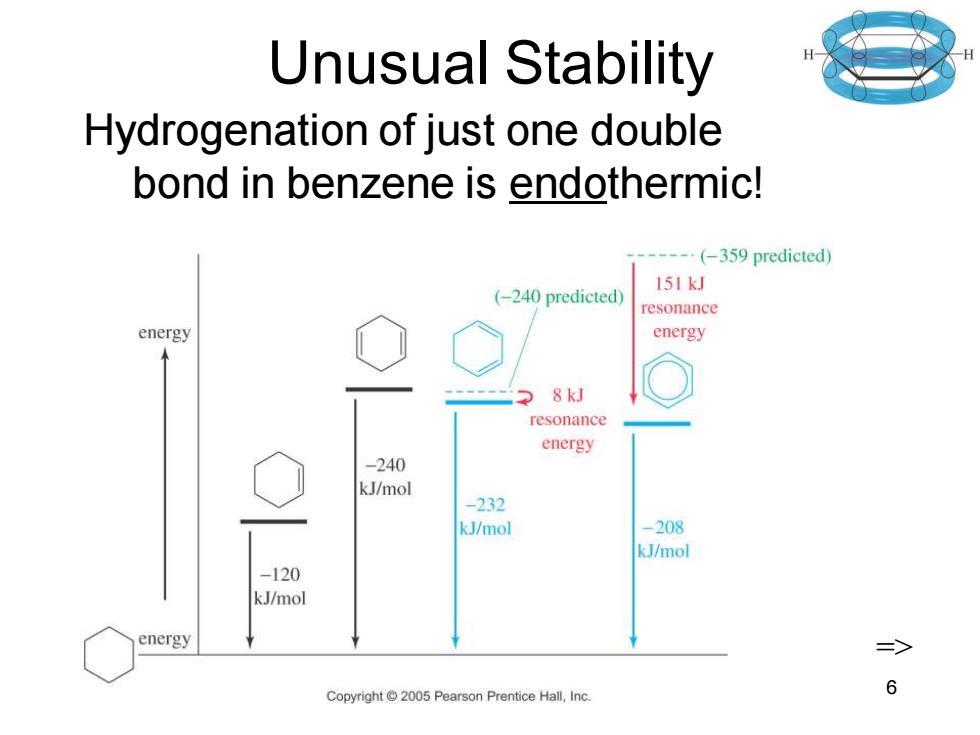
Unusual Stability Hydrogenation of just one double bond in benzene is endothermic! --…(-359 predicted) 151kJ (-240 predicted) resonance energy energy 8kJ resonance energy -240 kJ/mol -232 kJ/mol -208 kJ/mol -120 kJ/mol energy Copyright 2005 Pearson Prentice Hall,Inc. 6
Chapter 16 6 Unusual Stability Hydrogenation of just one double bond in benzene is endothermic! =>
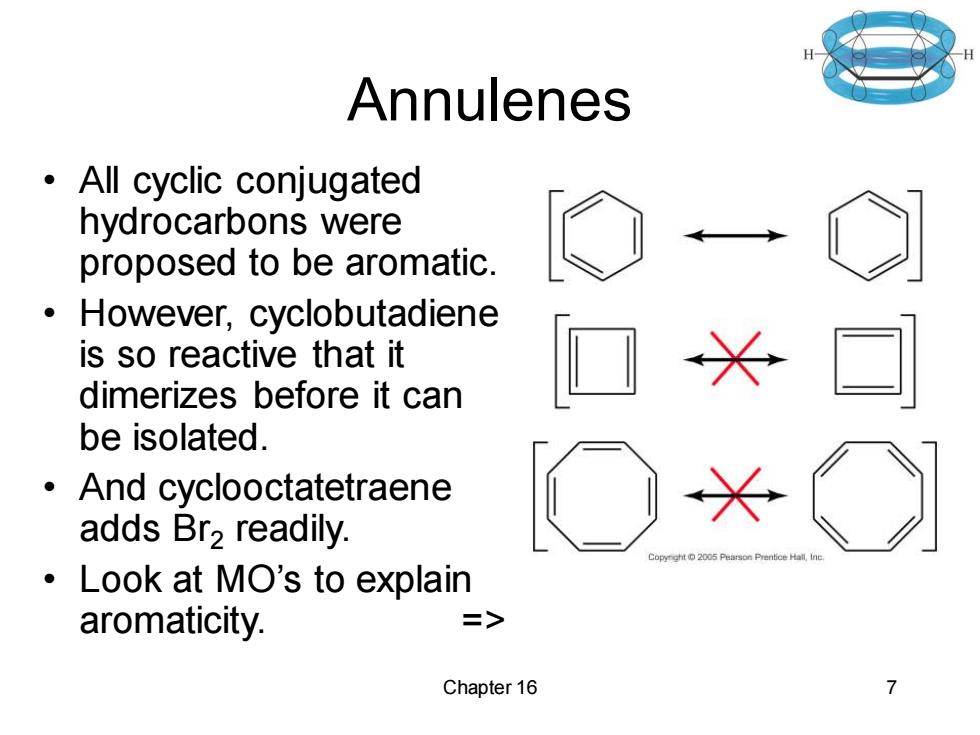
Annulenes All cyclic conjugated hydrocarbons were proposed to be aromatic. However,cyclobutadiene is so reactive that it dimerizes before it can be isolated. And cyclooctatetraene adds Br2 readily. Pea:son Prentice Hall, Look at MO's to explain aromaticity. => Chapter 16 7
Chapter 16 7 Annulenes • All cyclic conjugated hydrocarbons were proposed to be aromatic. • However, cyclobutadiene is so reactive that it dimerizes before it can be isolated. • And cyclooctatetraene adds Br2 readily. • Look at MO’s to explain aromaticity. =>

MO Rules for Benzene Six overlapping p orbitals must form six molecular orbitals. Three will be bonding,three antibonding. Lowest energy MO will have all bonding interactions,no nodes. As energy of MO increases,the number of nodes increases. 二> Chapter 16 8
Chapter 16 8 MO Rules for Benzene • Six overlapping p orbitals must form six molecular orbitals. • Three will be bonding, three antibonding. • Lowest energy MO will have all bonding interactions, no nodes. • As energy of MO increases, the number of nodes increases. =>
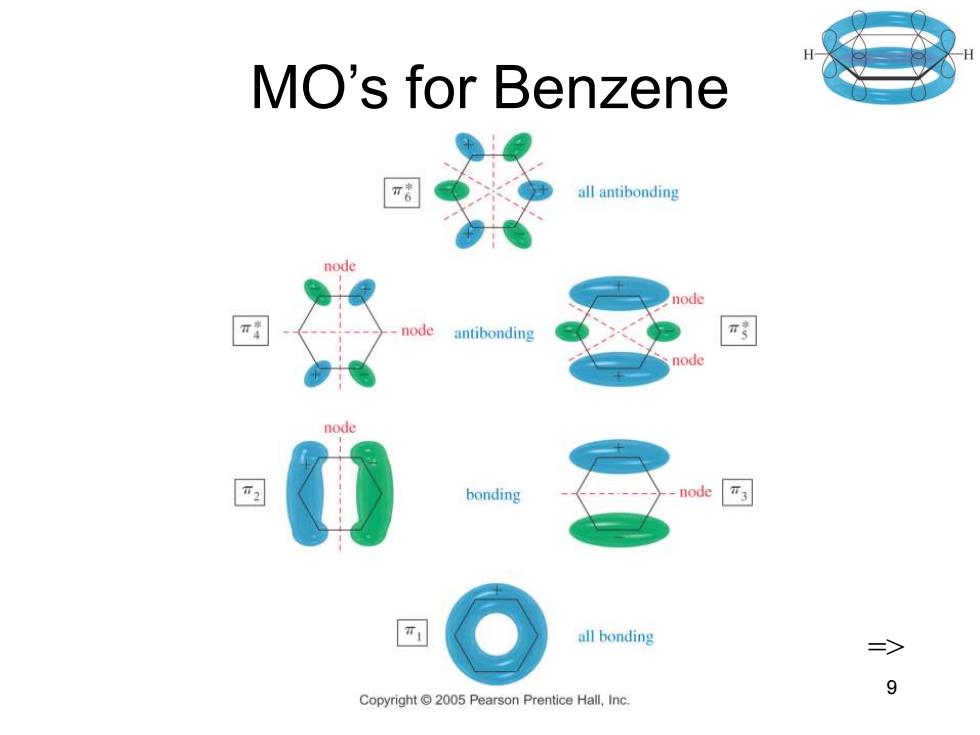
MO's for Benzene all antibonding node .node antibonding node bonding ode all bonding => 9 Copyright2005 Pearson Prentice Hall,Inc
Chapter 16 9 MO’s for Benzene =>
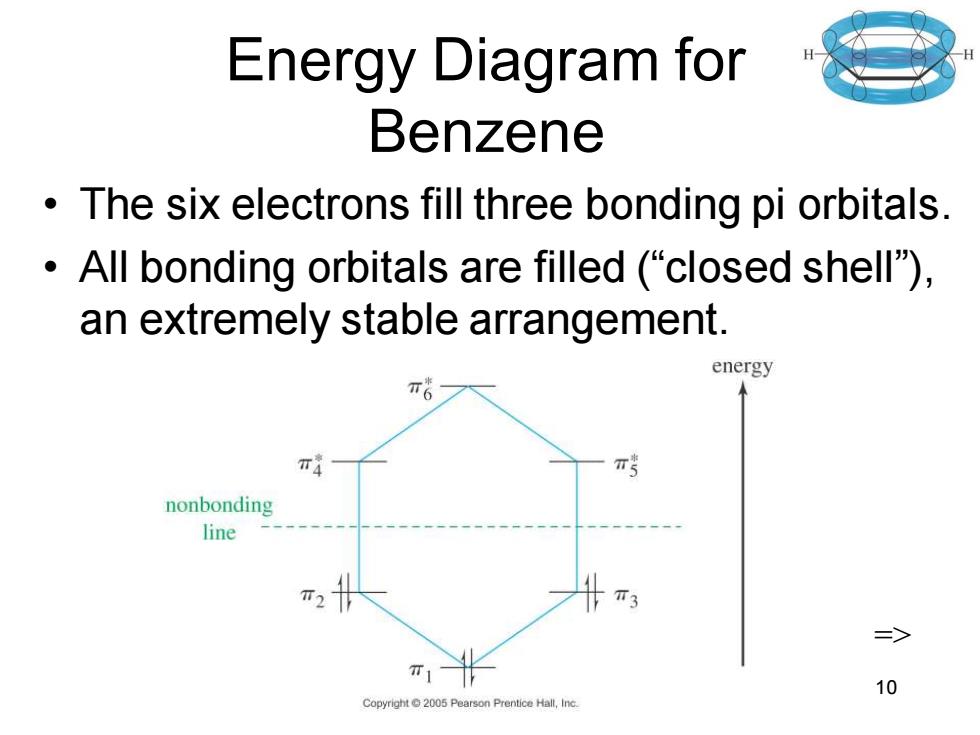
Energy Diagram for Benzene The six electrons fill three bonding pi orbitals. All bonding orbitals are filled ("closed shell). an extremely stable arrangement. energy π4 nonbonding line T3 三> 10 Copyright2005 Pearson Prentice Hall,Inc
Chapter 16 10 Energy Diagram for Benzene • The six electrons fill three bonding pi orbitals. • All bonding orbitals are filled (“closed shell”), an extremely stable arrangement. =>