
Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 22 Alpha Substitution and Condensations of Enols and Enolate lons Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2006,Prentice Hall
Chapter 22 Alpha Substitution and Condensations of Enols and Enolate Ions Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2006, Prentice Hall Organic Chemistry, 6th Edition L. G. Wade, Jr
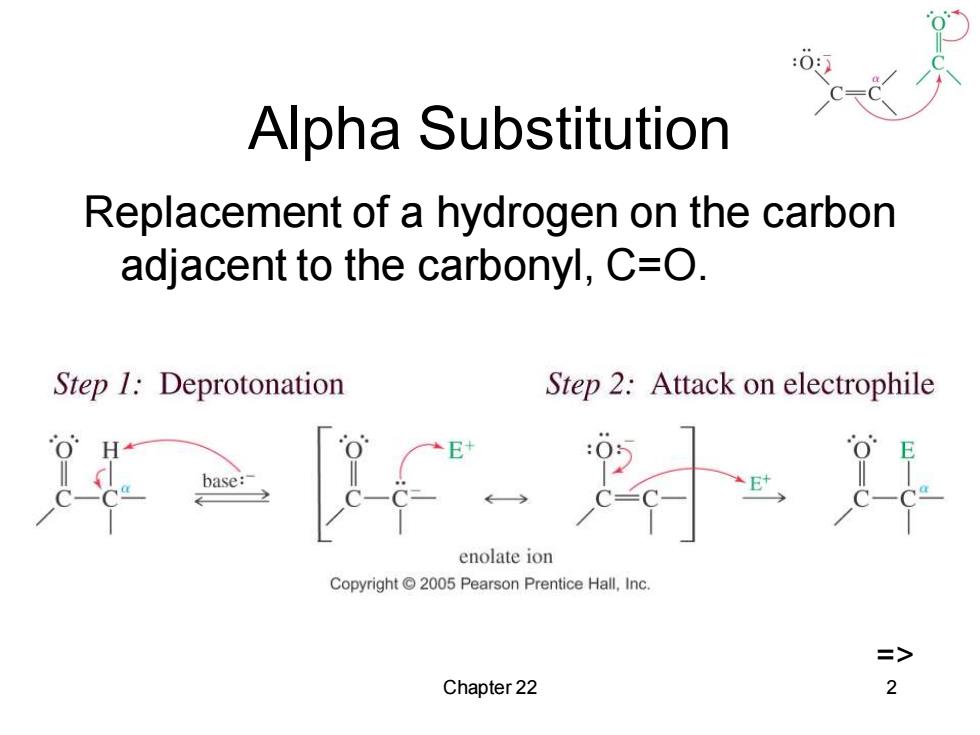
Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,C=O. Step 1:Deprotonation Step 2:Attack on electrophile base: enolate ion Copyright 2005 Pearson Prentice Hall,Inc. > Chapter 22 2
Chapter 22 2 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl, C=O. =>
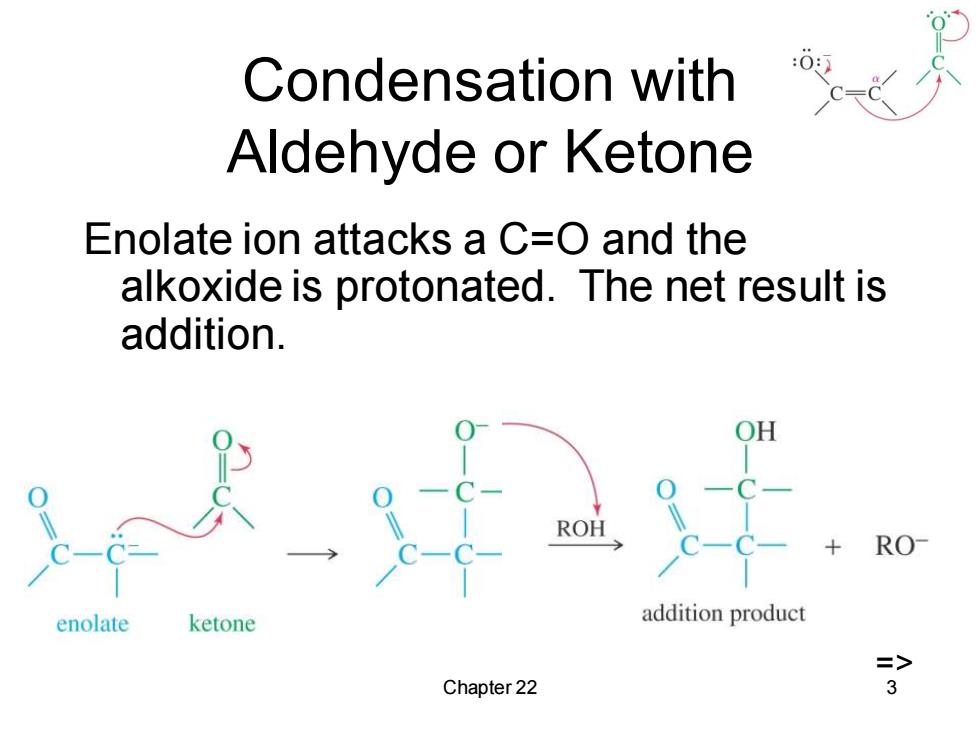
Condensation with Aldehyde or Ketone Enolate ion attacks a C=O and the alkoxide is protonated.The net result is addition. RO enolate ketone addition product => Chapter 22 3
Chapter 22 3 Condensation with Aldehyde or Ketone Enolate ion attacks a C=O and the alkoxide is protonated. The net result is addition. =>
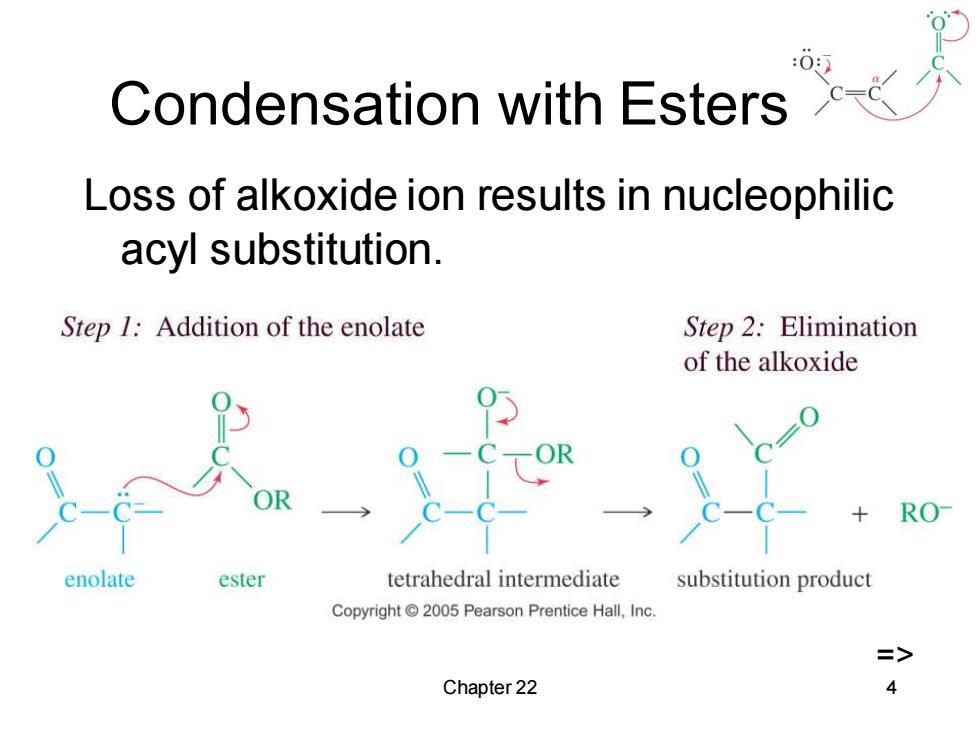
Condensation with Esters Loss of alkoxide ion results in nucleophilic acyl substitution. Step 1:Addition of the enolate Step 2:Elimination of the alkoxide RO enolate ester tetrahedral intermediate substitution product Copyright 2005 Pearson Prentice Hall,Inc. => Chapter 22 4
Chapter 22 4 Condensation with Esters Loss of alkoxide ion results in nucleophilic acyl substitution. =>
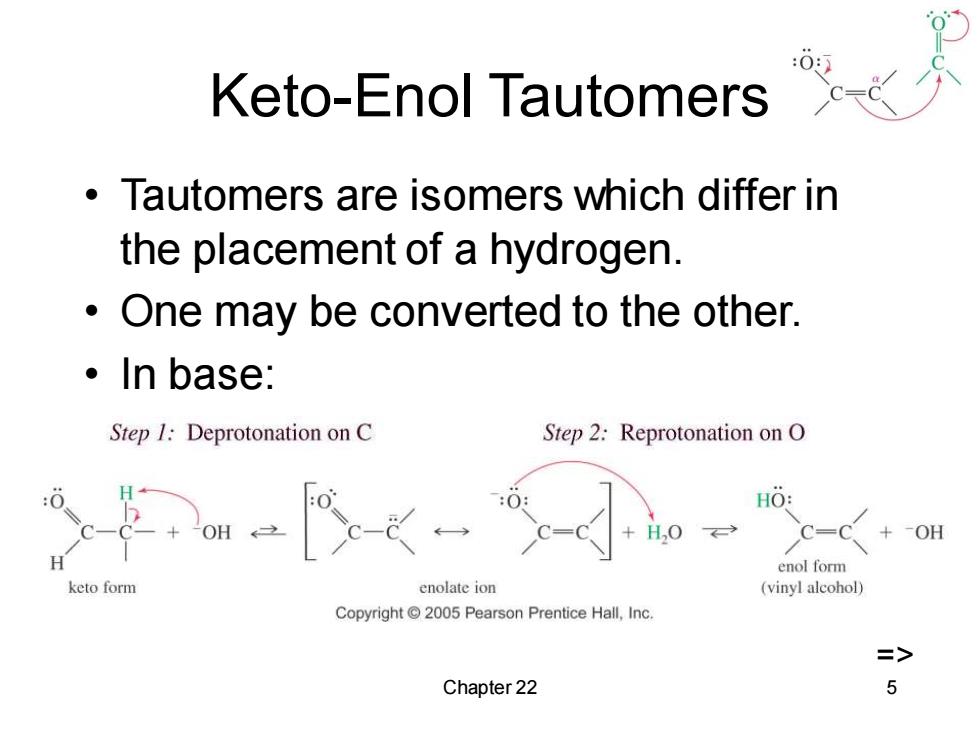
Keto-Enol Tautomers Tautomers are isomers which differ in the placement of a hydrogen. One may be converted to the other. ·In base: Step 1:Deprotonation on C Step 2:Reprotonation on O HO: OH +OH H enol form keto form enolate ion (vinyl alcohol) Copyright2005 Pearson Prentice Hall,Inc. => Chapter 22 5
Chapter 22 5 Keto-Enol Tautomers • Tautomers are isomers which differ in the placement of a hydrogen. • One may be converted to the other. • In base: =>
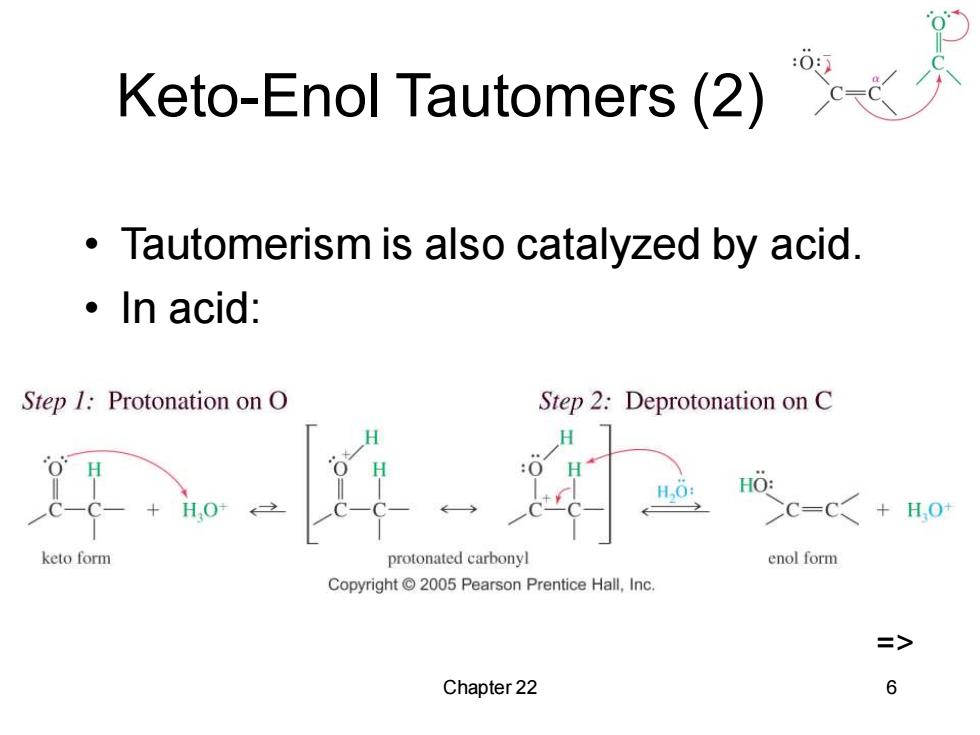
Keto-Enol Tautomers(2) Tautomerism is also catalyzed by acid. ·In acid: Step 1:Protonation on O Step 2:Deprotonation on C H H.O: HO: HO+ >c=C+H,0 keto form protonated carbonyl enol form Copyright 2005 Pearson Prentice Hall,Inc. => Chapter 22 6
Chapter 22 6 Keto-Enol Tautomers (2) • Tautomerism is also catalyzed by acid. • In acid: =>
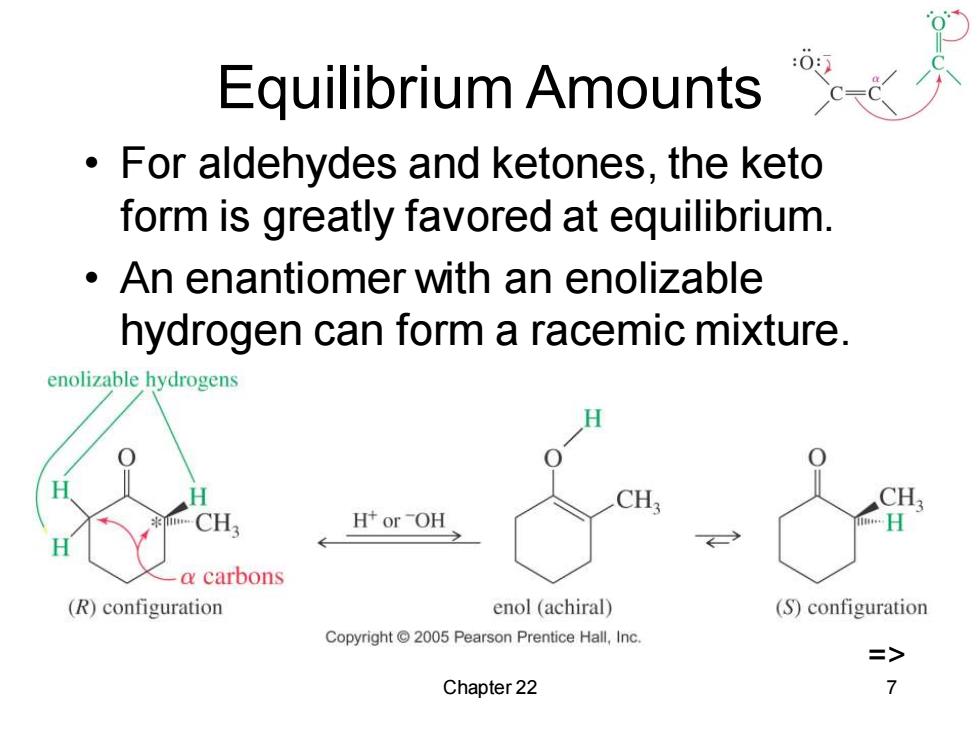
Equilibrium Amounts For aldehydes and ketones,the keto form is greatly favored at equilibrium. An enantiomer with an enolizable hydrogen can form a racemic mixture. enolizable hydrogens 1 CH H+orOH H a carbons (R)configuration enol (achiral) (S)configuration Copyright 2005 Pearson Prentice Hall,Inc. => Chapter 22 7
Chapter 22 7 Equilibrium Amounts • For aldehydes and ketones, the keto form is greatly favored at equilibrium. • An enantiomer with an enolizable hydrogen can form a racemic mixture. =>
Acidity of a-Hydrogens pKa for a-H of aldehyde or ketone ~20. Much more acidic than alkane or alkene (pKa 40)or alkyne (pKa 25). Less acidic than water(pKa =15.7)or alcohol (pKa 16-19). In the presence of hydroxide or alkoxide ions,only a small amount of enolate ion is present at equilibrium. => Chapter 22 8
Chapter 22 8 Acidity of -Hydrogens • pKa for -H of aldehyde or ketone ~20. • Much more acidic than alkane or alkene (pKa > 40) or alkyne (pKa = 25). • Less acidic than water (pKa = 15.7) or alcohol (pKa = 16-19). • In the presence of hydroxide or alkoxide ions, only a small amount of enolate ion is present at equilibrium. =>
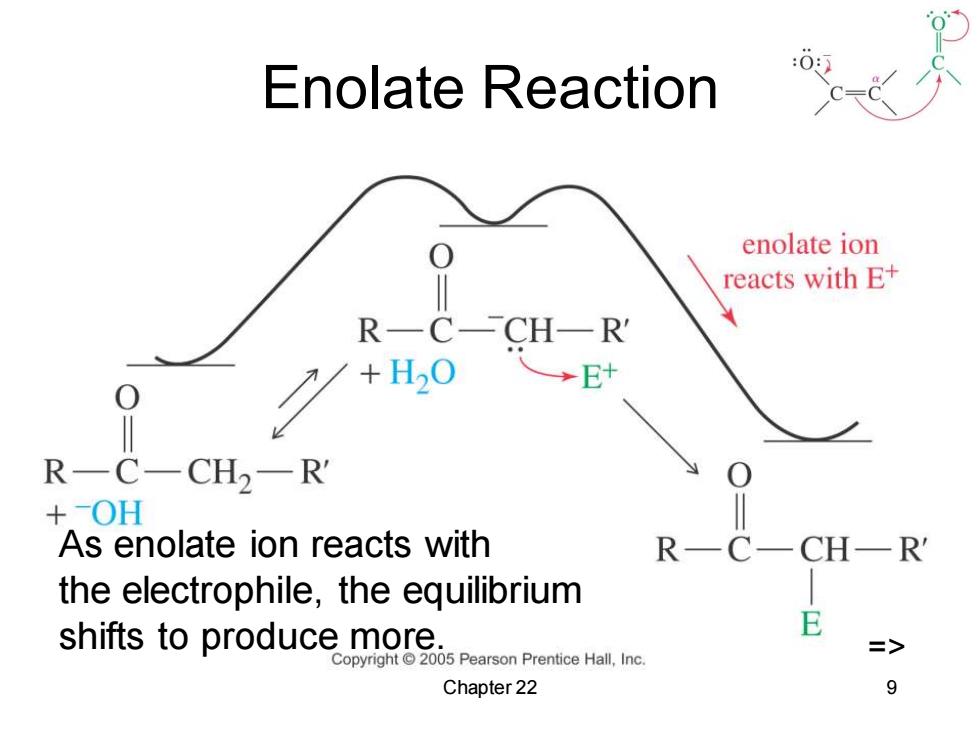
Enolate Reaction enolate ion reacts with E+ RC-CHR →E+ R一C一CH2-R +OH As enolate ion reacts with RC CHR the electrophile,the equilibrium shifts to produce more. E Copyright 2005 Pearson Prentice Hall,Inc. Chapter 22 9
Chapter 22 9 Enolate Reaction => As enolate ion reacts with the electrophile, the equilibrium shifts to produce more
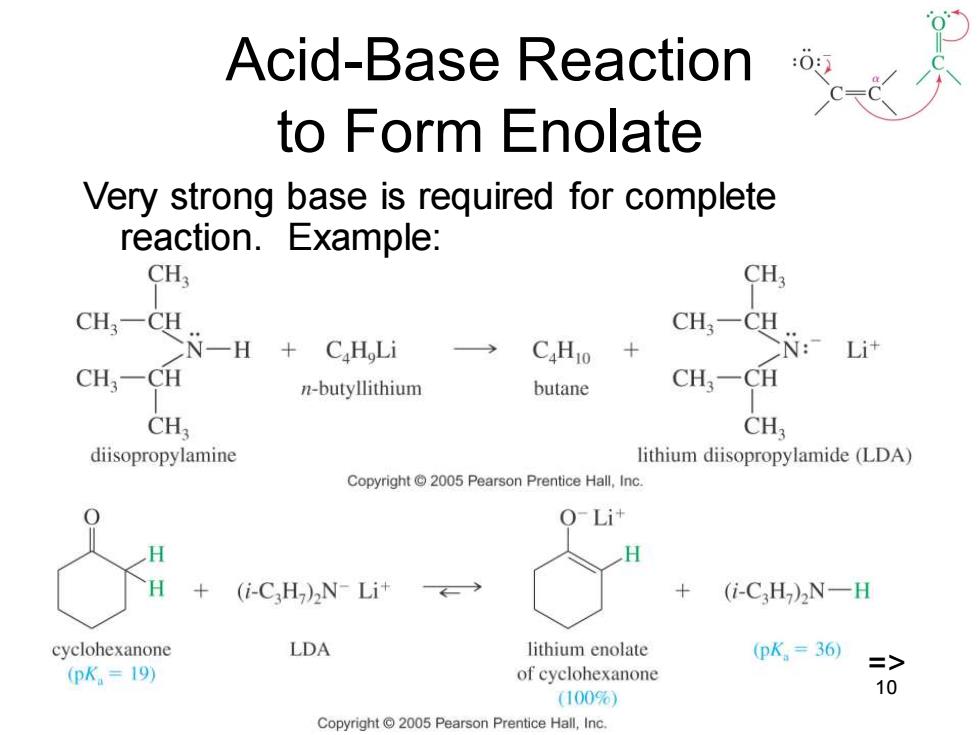
Acid-Base Reaction to Form Enolate Very strong base is required for complete reaction.Example: CHs CH, CH,一C CH一C N-一H C.H Li CaH10 CN: Li计 CH3-CH n-butyllithium butane CH:-CH CH CH; diisopropylamine lithium diisopropylamide (LDA) Copyright 2005 Pearson Prentice Hall,Inc. O-Lit (i-CH)N-Li+ (i-C,H)2N-H cyclohexanone LDA lithium enolate (pK,=36 (pK.=I19) => of cyclohexanone 10 (100%) Copyright 2005 Pearson Prentice Hall,Inc
Chapter 22 10 Acid-Base Reaction to Form Enolate Very strong base is required for complete reaction. Example: =>