Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 2 Structure and Properties of Organic Molecules Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2006,Prentice Hall
Chapter 2 Structure and Properties of Organic Molecules Organic Chemistry, 6th Edition L. G. Wade, Jr. Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2006, Prentice Hall
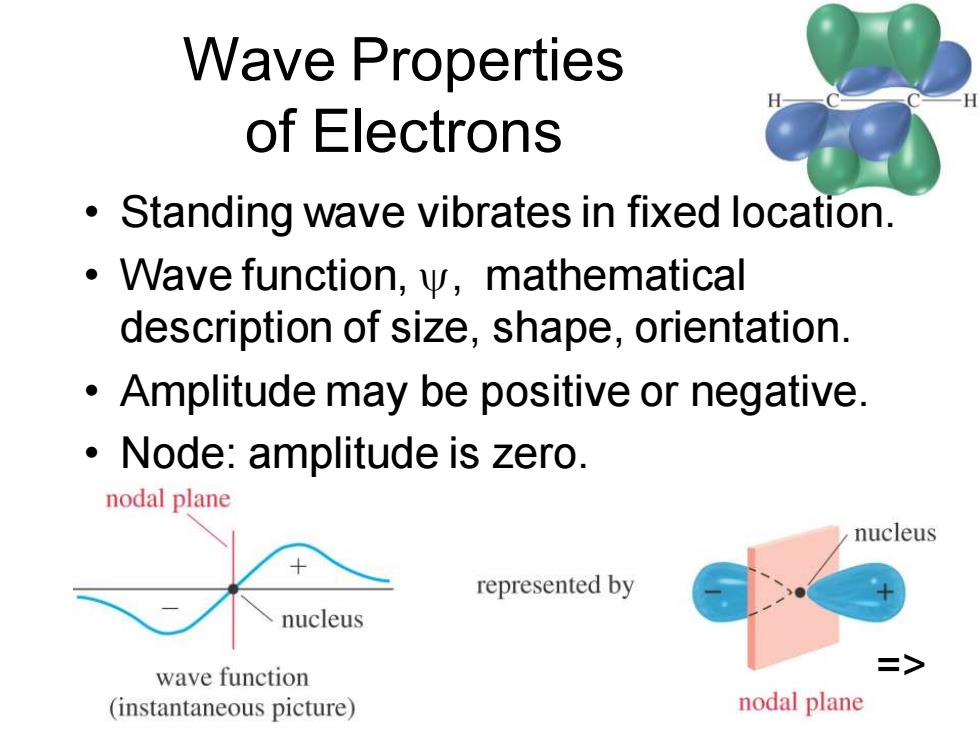
Wave Properties of Electrons Standing wave vibrates in fixed location. Wave function,y,mathematical description of size,shape,orientation. Amplitude may be positive or negative. Node:amplitude is zero. nodal plane nucleus represented by nucleus wave function > (instantaneous picture) nodal plane
Chapter 2 2 Wave Properties of Electrons • Standing wave vibrates in fixed location. • Wave function, , mathematical description of size, shape, orientation. • Amplitude may be positive or negative. • Node: amplitude is zero. =>
Wave Interactions Linear combination of atomic orbitals >on different atoms produce molecular orbitals >on the same atom give hybrid orbitals. Conservation of orbitals. Waves that are in phase add together. Amplitude increases. Waves that are out of phase cancel out. Chapter 2 3
Chapter 2 3 Wave Interactions • Linear combination of atomic orbitals ➢on different atoms produce molecular orbitals ➢on the same atom give hybrid orbitals. • Conservation of orbitals. • Waves that are in phase add together. Amplitude increases. • Waves that are out of phase cancel out. =>
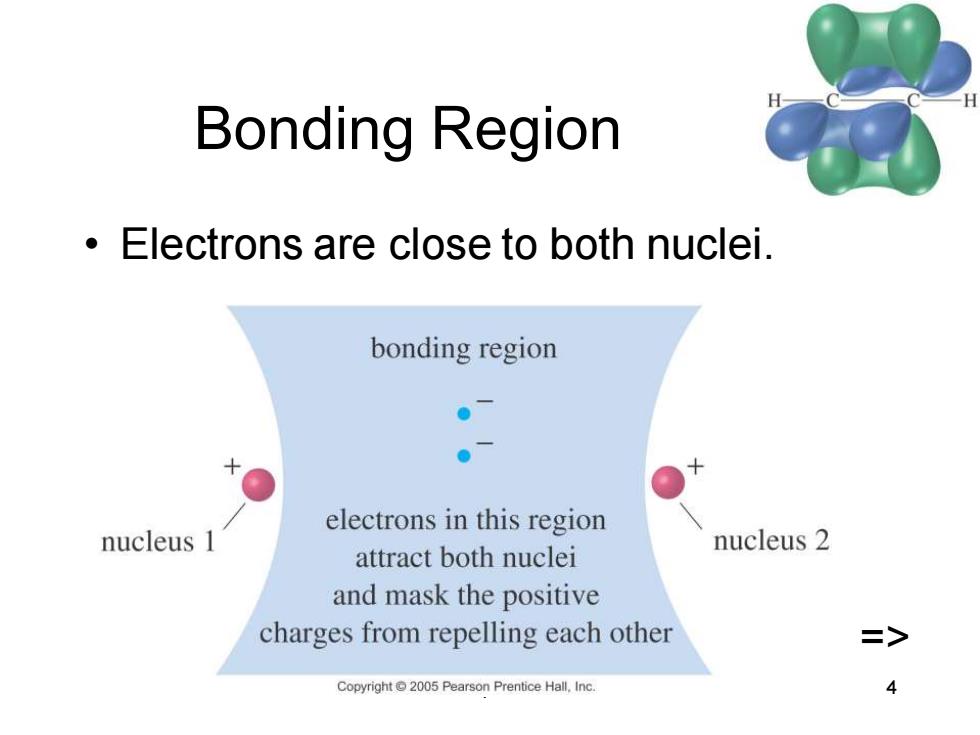
Bonding Region Electrons are close to both nuclei. bonding region ●入 electrons in this region nucleus 1 attract both nuclei nucleus 2 and mask the positive charges from repelling each other > Copyright2005 Pearson Prentice Hall.Inc
Chapter 2 4 Bonding Region • Electrons are close to both nuclei. =>
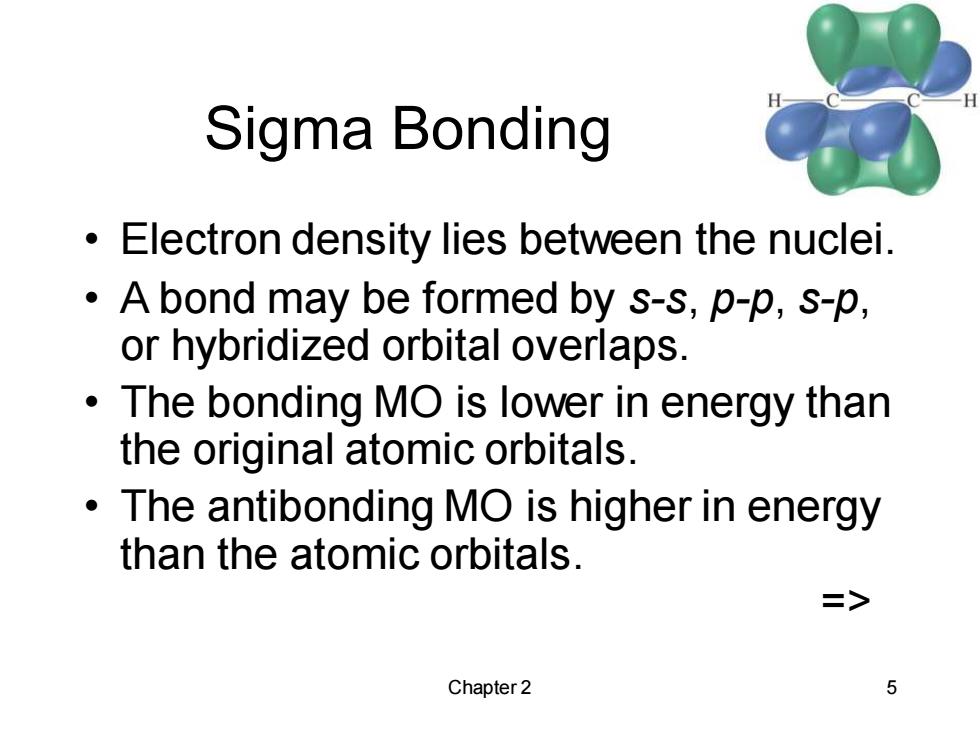
Sigma Bonding Electron density lies between the nuclei. A bond may be formed by s-s,p-p,s-p, or hybridized orbital overlaps. The bonding MO is lower in energy than the original atomic orbitals. The antibonding MO is higher in energy than the atomic orbitals. => Chapter 2 5
Chapter 2 5 Sigma Bonding • Electron density lies between the nuclei. • A bond may be formed by s-s, p-p, s-p, or hybridized orbital overlaps. • The bonding MO is lower in energy than the original atomic orbitals. • The antibonding MO is higher in energy than the atomic orbitals. =>

Bonding Molecular Orbital Two hydrogens,1s constructive overlap Constructive Interaction:The two Is orbitals are in phase and have the same sign add bonding molecular orbital represented by: => σ-bonding MO 6 Copyright2005 Pearson Prentice Hall,Inc
Chapter 2 6 Bonding Molecular Orbital Two hydrogens, 1s constructive overlap =>
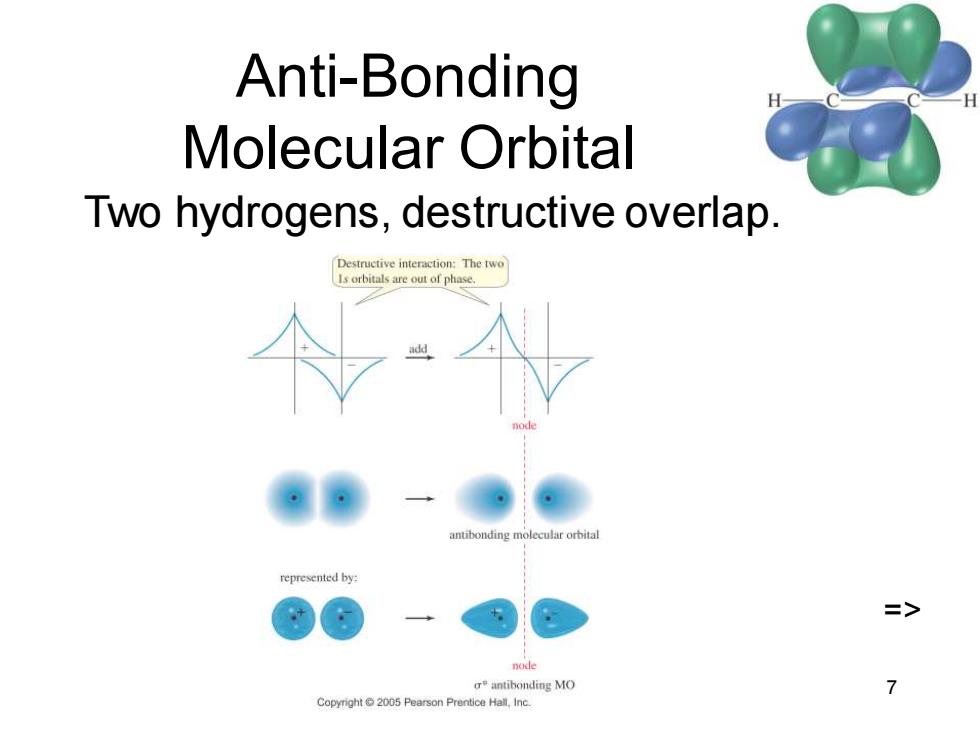
Anti-Bonding Molecular Orbital Two hydrogens,destructive overlap. Destructive interaction:The twe 1s orbitals are out of phase. anubonding molecular orbital represented by: => node antibonding MO 7 Pearson Prentice Hall,Inc
Chapter 2 7 Anti-Bonding Molecular Orbital Two hydrogens, destructive overlap. =>
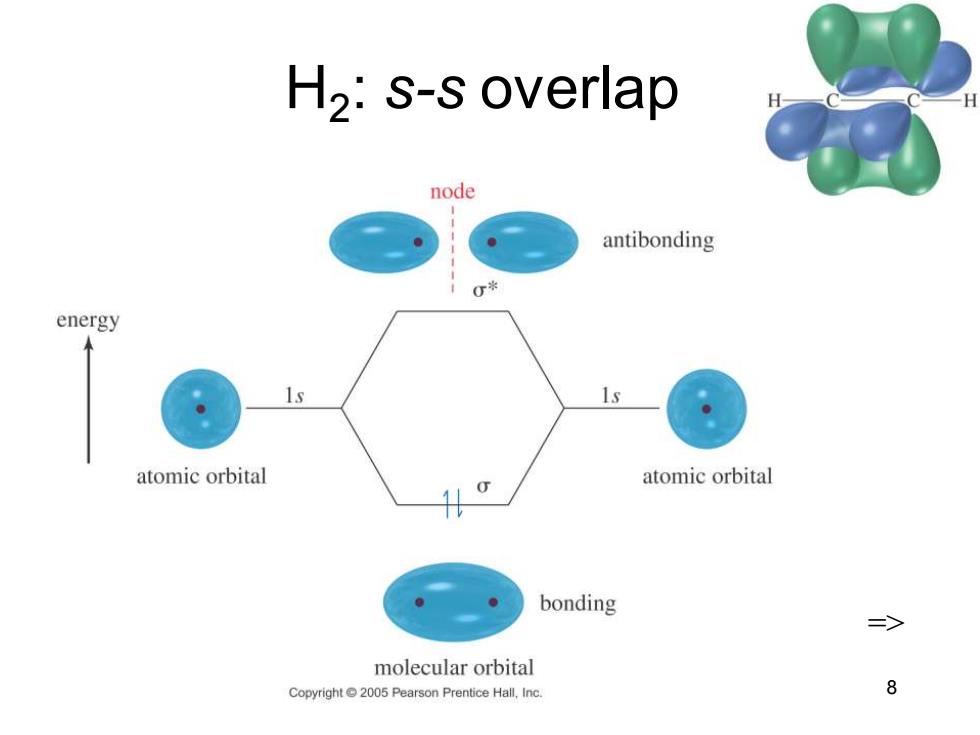
H2:s-s overlap node antibonding 0* energy atomic orbital atomic orbital bonding 三> molecular orbital Copyright 2005 Pearson Prentice Hall,Inc. 8
Chapter 2 8 H2 : s-s overlap =>
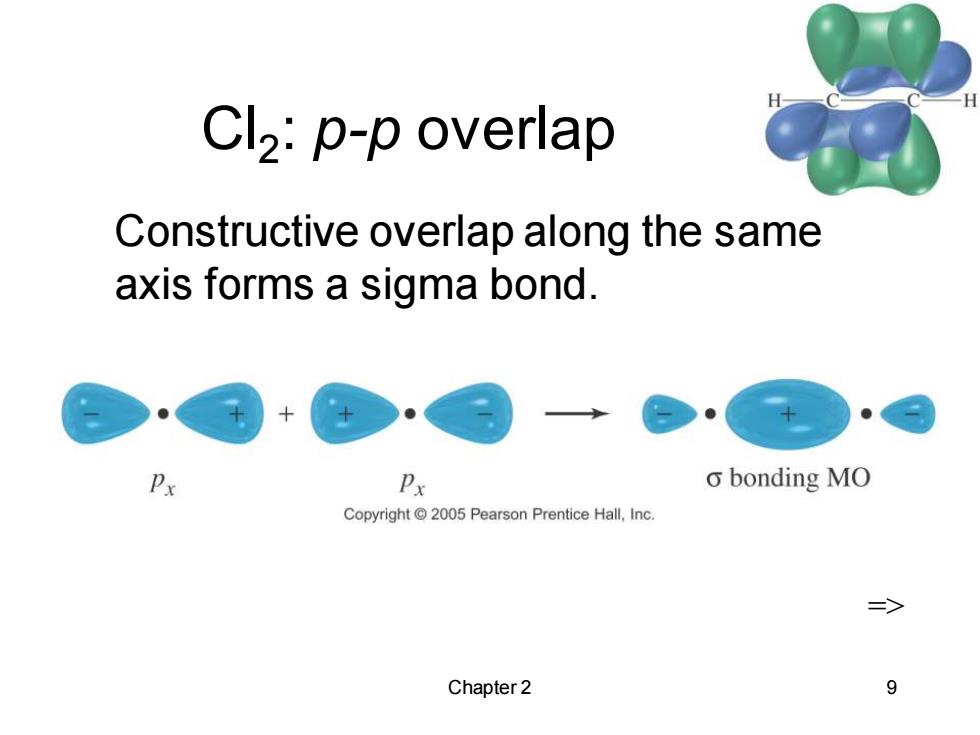
Cl2:p-p overlap Constructive overlap along the same axis forms a sigma bond. Px Px o bonding MO Copyright 2005 Pearson Prentice Hall,Inc. => Chapter 2 9
Chapter 2 9 Cl2 : p-p overlap => Constructive overlap along the same axis forms a sigma bond
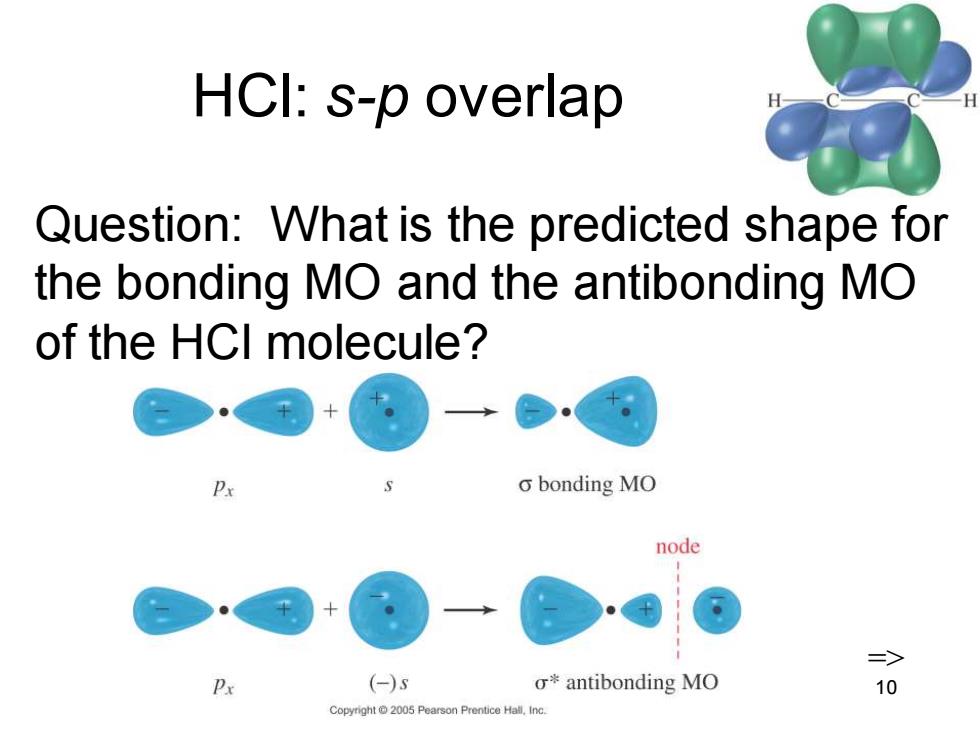
HCI:s-p overlap Question:What is the predicted shape for the bonding MO and the antibonding MO of the HCI molecule? o bonding MO node => Px (-)s o*antibonding MO 10 Copyright 2005 Pearson Prentice Hall.Inc
Chapter 2 10 HCl: s-p overlap Question: What is the predicted shape for the bonding MO and the antibonding MO of the HCl molecule? =>