Organic Chemistry,6th Edition H.. L.G.Wade,Jr. sP: p H Chapter 4 The Study of Chemical Reactions Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2006,Prentice Hall
Chapter 4 The Study of Chemical Reactions Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2006, Prentice Hall Organic Chemistry, 6th Edition L. G. Wade, Jr

Introduction H. H ·Reactants-→Products:overall reaction. Mechanism:step-by-step pathway. To learn more about a reaction: >Thermodynamics Kinetics. => Chapter 4 2
Chapter 4 2 Introduction • Reactants → Products: overall reaction. • Mechanism: step-by-step pathway. • To learn more about a reaction: ➢Thermodynamics ➢ Kinetics. =>
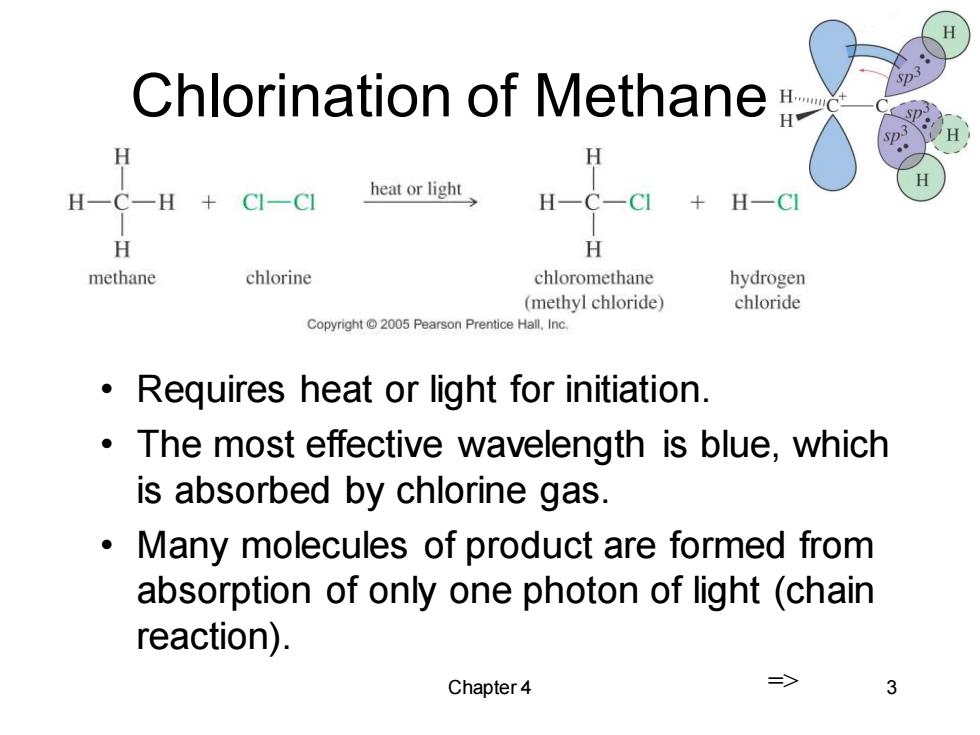
Chlorination of Methane H H H一C一H CI-CI heat or light H-CI H H methane chlorine chloromethane hydrogen (methyl chloride) chloride Copyright 2005 Pearson Prentice Hall,Inc. 。 Requires heat or light for initiation. The most effective wavelength is blue,which is absorbed by chlorine gas. 。 Many molecules of product are formed from absorption of only one photon of light (chain reaction). Chapter 4 => 3
Chapter 4 3 Chlorination of Methane • Requires heat or light for initiation. • The most effective wavelength is blue, which is absorbed by chlorine gas. • Many molecules of product are formed from absorption of only one photon of light (chain reaction). =>
The Free-Radical H Chain Reaction Initiation generates a reactive intermediate. Propagation:the intermediate reacts with a stable molecule to produce another reactive intermediate (and a product molecule). Termination:side reactions that destroy the reactive intermediate. > Chapter4 4
Chapter 4 4 The Free-Radical Chain Reaction • Initiation generates a reactive intermediate. • Propagation: the intermediate reacts with a stable molecule to produce another reactive intermediate (and a product molecule). • Termination: side reactions that destroy the reactive intermediate. =>
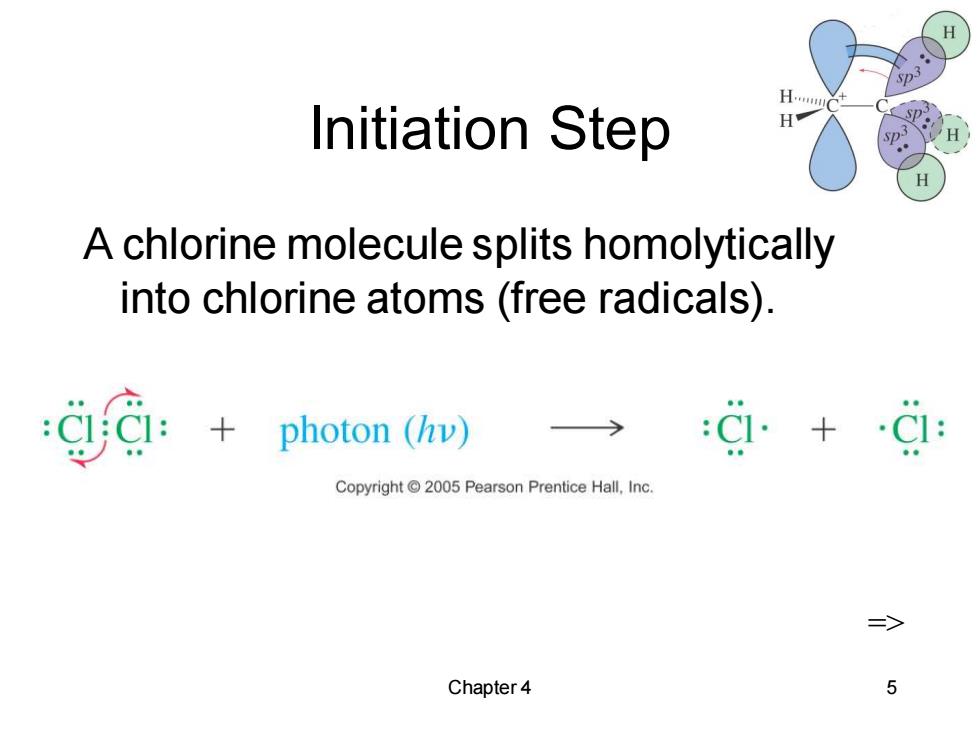
Initiation Step H. H A chlorine molecule splits homolytically into chlorine atoms(free radicals). CC photon (hv) +C: Copyright2005 Pearson Prentice Hall.Inc. Chapter 4 5
Chapter 4 5 Initiation Step A chlorine molecule splits homolytically into chlorine atoms (free radicals). =>
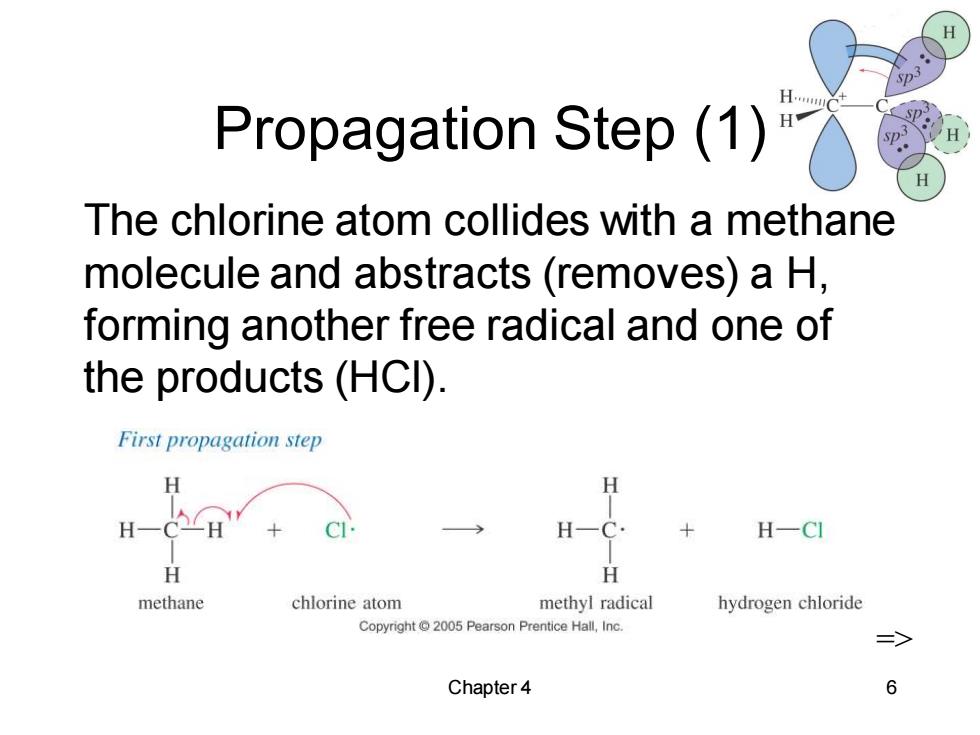
H Propagation Step (1) The chlorine atom collides with a methane molecule and abstracts (removes)a H, forming another free radical and one of the products (HCI). First propagation step H H H-CE H-C. H-CI H H methane chlorine atom methyl radical hydrogen chloride Copyright 2005 Pearson Prentice Hall,Inc. 三> Chapter 4 6
Chapter 4 6 Propagation Step (1) The chlorine atom collides with a methane molecule and abstracts (removes) a H, forming another free radical and one of the products (HCl). =>
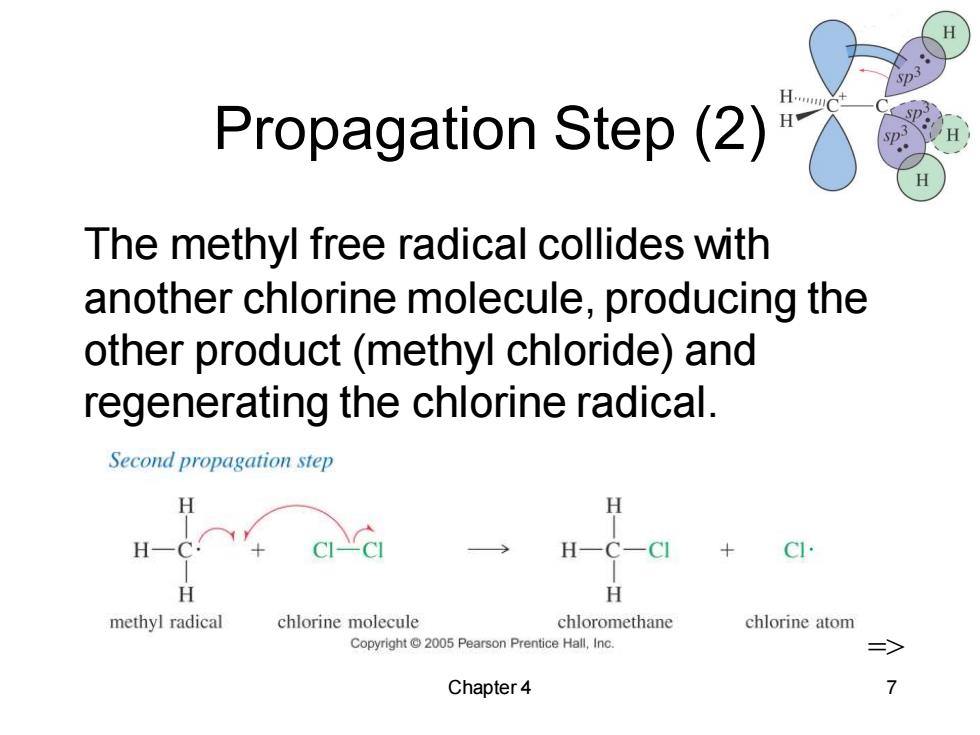
H Propagation Step(2) p H The methyl free radical collides with another chlorine molecule,producing the other product(methyl chloride)and regenerating the chlorine radical. Second propagation step H H H methyl radical chlorine molecule chloromethane chlorine atom Copyright@2005 Pearson Prentice Hall,Inc. Chapter 4
Chapter 4 7 Propagation Step (2) The methyl free radical collides with another chlorine molecule, producing the other product (methyl chloride) and regenerating the chlorine radical. =>
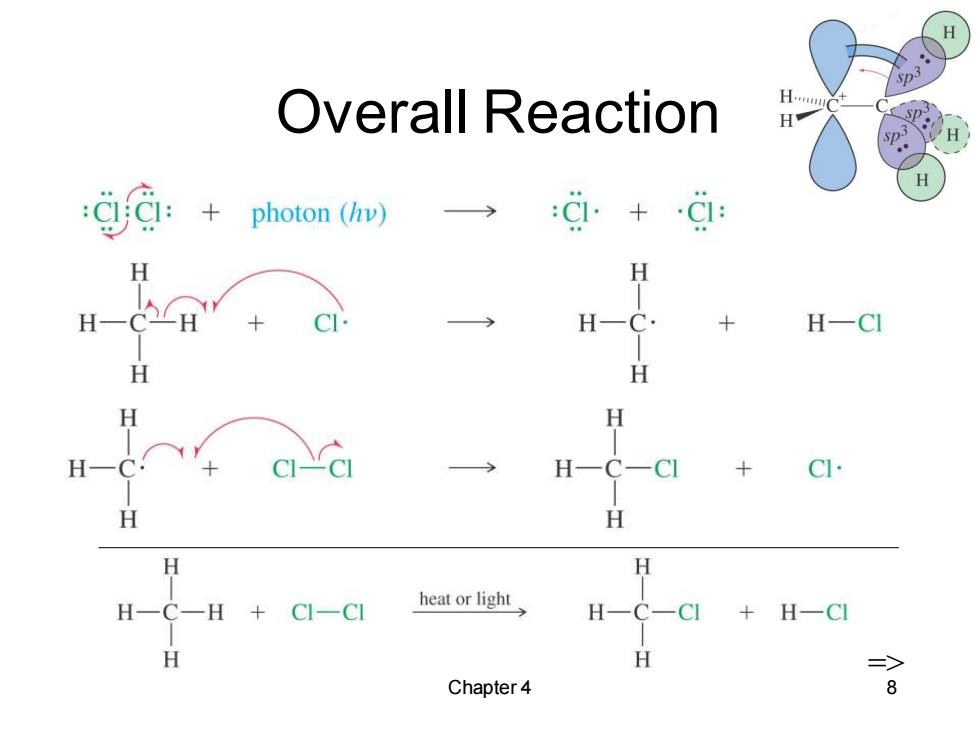
Overall Reaction H.. H g:+ photon(hv) Ci. H H H H-C· H一CI H H H H CI- H H H H C-H CI-CI heat or light +H一C H => Chapter 4 8
Chapter 4 8 Overall Reaction =>
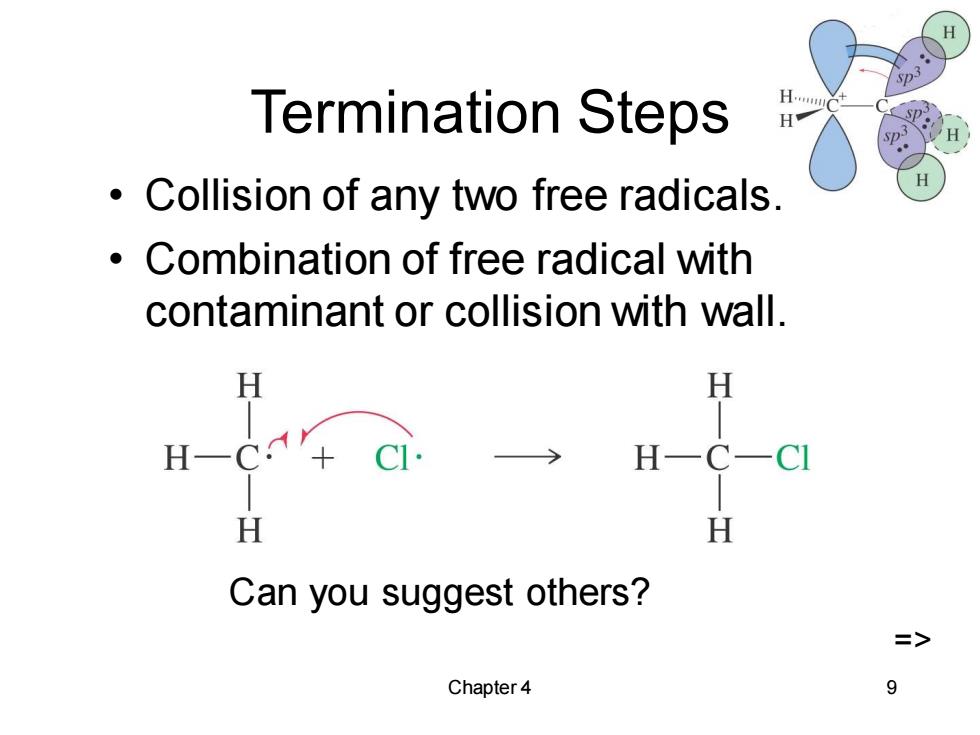
Termination Steps H.. sP: Collision of any two free radicals. H Combination of free radical with contaminant or collision with wall. H H H H H H Can you suggest others? => Chapter 4 9
Chapter 4 9 Termination Steps • Collision of any two free radicals. • Combination of free radical with contaminant or collision with wall. Can you suggest others? =>
H. Equilibrium Constant ·Kea=[products] [reactants] For chlorination Keg=1.1 x 1019 ·Large value indicates reaction“goes to completion.” => Chapter 4 10
Chapter 4 10 Equilibrium Constant • Keq = [products] [reactants] • For chlorination Keq = 1.1 x 1019 • Large value indicates reaction “goes to completion.” =>