
Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 14 Ethers,Epoxides, and Sulfides Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2006,Prentice Hall
Chapter 14 Ethers, Epoxides, and Sulfides Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2006, Prentice Hall Organic Chemistry, 6th Edition L. G. Wade, Jr
Introduction Formula R-O-R'where R and R'are alkyl or aryl. Symmetrical or unsymmetrical 。Examples: CH3-O-CH3 )-CH3 => Chapter 14 2
Chapter 14 2 Introduction • Formula R-O-R where R and R are alkyl or aryl. • Symmetrical or unsymmetrical • Examples: O CH3 CH3 O CH3 O =>
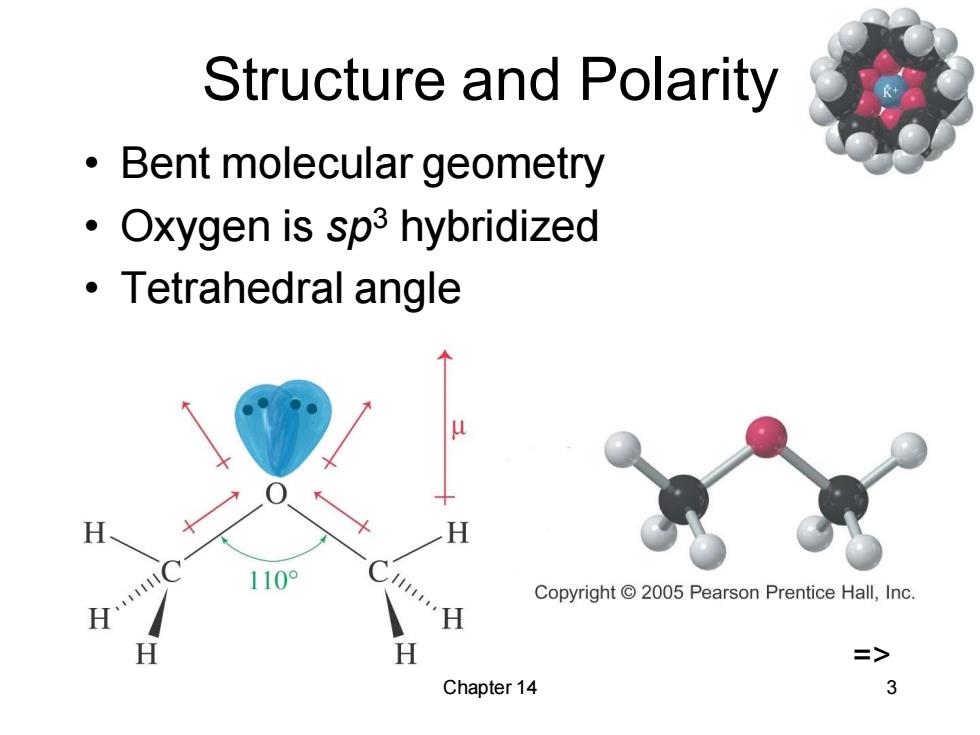
Structure and Polarity Bent molecular geometry Oxygen is sp3 hybridized 。Tetrahedral angle Copyright 2005 Pearson Prentice Hall,Inc. H => Chapter 14 3
Chapter 14 3 Structure and Polarity • Bent molecular geometry • Oxygen is sp3 hybridized • Tetrahedral angle =>
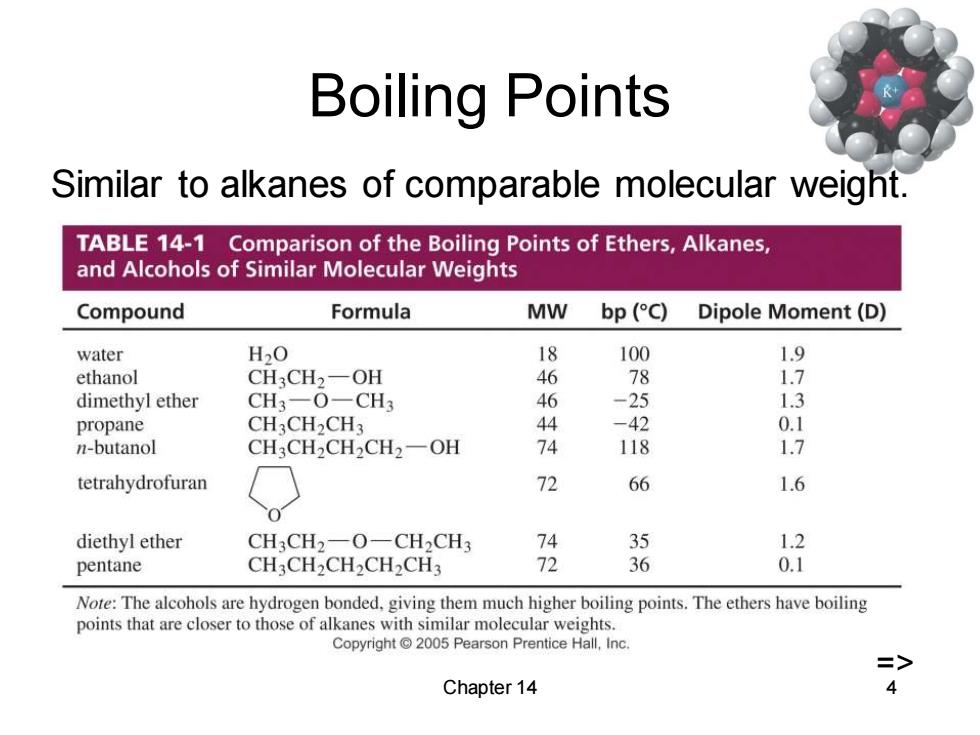
Boiling Points Similar to alkanes of comparable molecular weight. TABLE 14-1 Comparison of the Boiling Points of Ethers,Alkanes, and Alcohols of Similar Molecular Weights Compound Formula MW bp (C) Dipole Moment(D) water H20 18 100 1.9 ethanol CH3CH2-OH 46 78 1.7 dimethyl ether CH3一O-CH3 46 -25 1.3 propane CH3CH2CH3 44 -42 0.1 n-butanol CH3CH2CH2CH2一OH 74 118 1.7 tetrahydrofuran 72 66 1.6 diethyl ether CH3CH2-O-CH2CH3 74 35 1.2 pentane CH3CH2CH2CH2CH3 72 6 0.1 Note:The alcohols are hydrogen bonded.giving them much higher boiling points.The ethers have boiling points that are closer to those of alkanes with similar molecular weights. Copyright 2005 Pearson Prentice Hall,Inc. => Chapter 14 4
Chapter 14 4 Boiling Points Similar to alkanes of comparable molecular weight. =>
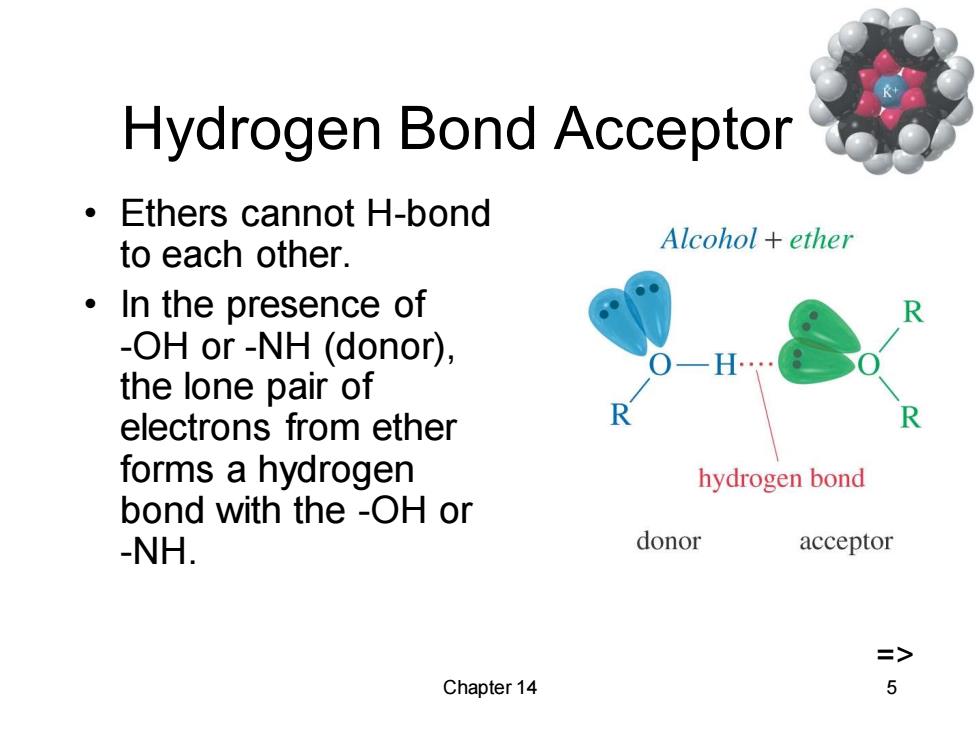
Hydrogen Bond Acceptor Ethers cannot H-bond to each other. Alcohol ether ·In the presence of -OH or-NH (donor), the lone pair of electrons from ether forms a hydrogen hydrogen bond bond with the -OH or -NH. donor acceptor => Chapter 14 5
Chapter 14 5 Hydrogen Bond Acceptor • Ethers cannot H-bond to each other. • In the presence of -OH or -NH (donor), the lone pair of electrons from ether forms a hydrogen bond with the -OH or -NH. =>
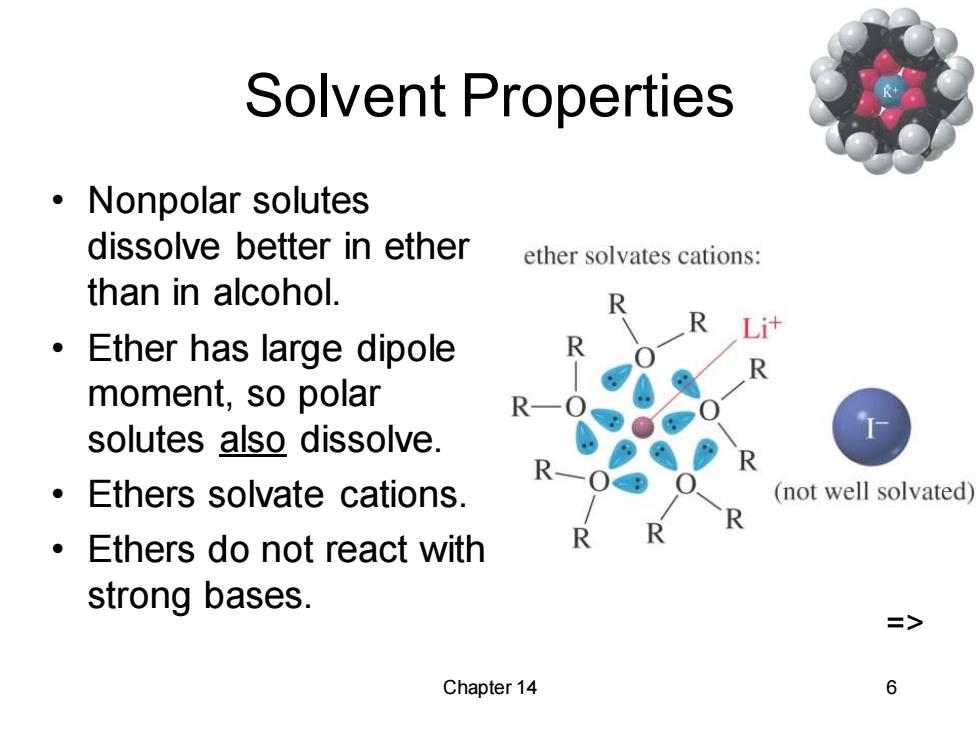
Solvent Properties ·Nonpolar solutes dissolve better in ether ether solvates cations: than in alcohol. 0 Ether has large dipole moment,so polar R solutes also dissolve. Ethers solvate cations. (not well solvated) Ethers do not react with strong bases. => Chapter 14 6
Chapter 14 6 Solvent Properties • Nonpolar solutes dissolve better in ether than in alcohol. • Ether has large dipole moment, so polar solutes also dissolve. • Ethers solvate cations. • Ethers do not react with strong bases. =>
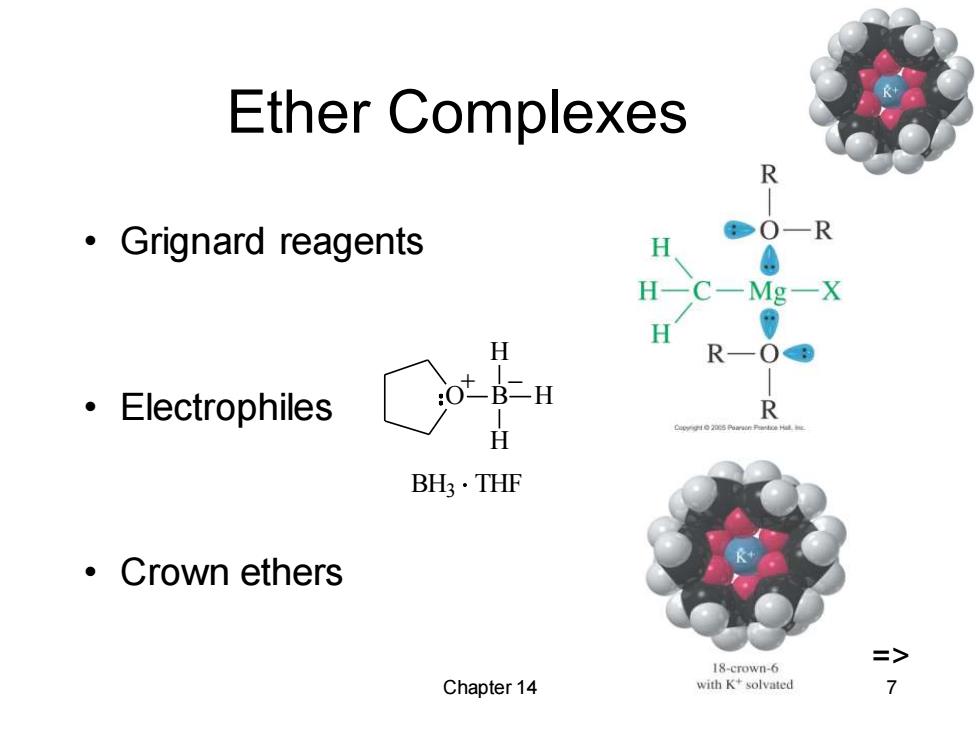
Ether Complexes R ·Grignard reagents O-R H H-C一Mg-X H H R—O8 ·Electrophiles :OtB二H R H BH3·THF ·Crown ethers 18-crown-6 Chapter 14 with K+solvated 7
Chapter 14 7 Ether Complexes • Grignard reagents • Electrophiles • Crown ethers O B H H H + _ BH3 THF =>
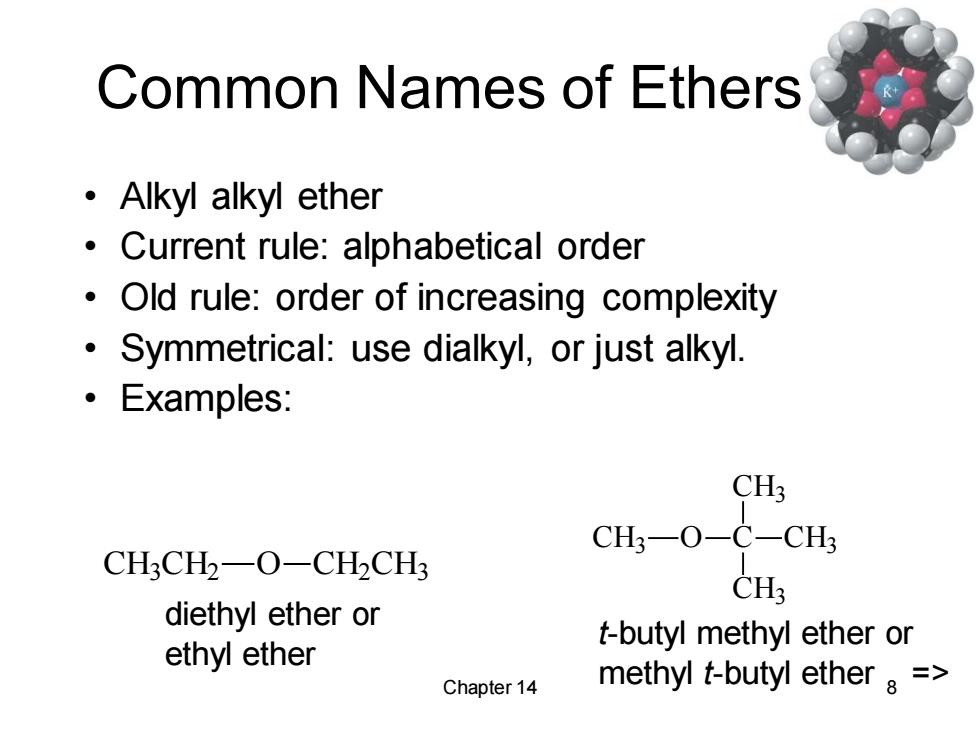
Common Names of Ethers ·Alkyl alkyl ether Current rule:alphabetical order Old rule:order of increasing complexity 。 Symmetrical:use dialkyl,or just alkyl. ·Examples: CH3 CH3一O-C-CH3 CH3CH2-O-CH2CH3 CH3 diethyl ether or t-butyl methyl ether or ethyl ether Chapter 14 methyl t-butyl ether a =
Chapter 14 8 Common Names of Ethers • Alkyl alkyl ether • Current rule: alphabetical order • Old rule: order of increasing complexity • Symmetrical: use dialkyl, or just alkyl. • Examples: CH3 CH2 O CH2 CH3 diethyl ether or ethyl ether CH3 O C CH3 CH3 CH3 t-butyl methyl ether or methyl t-butyl ether =>
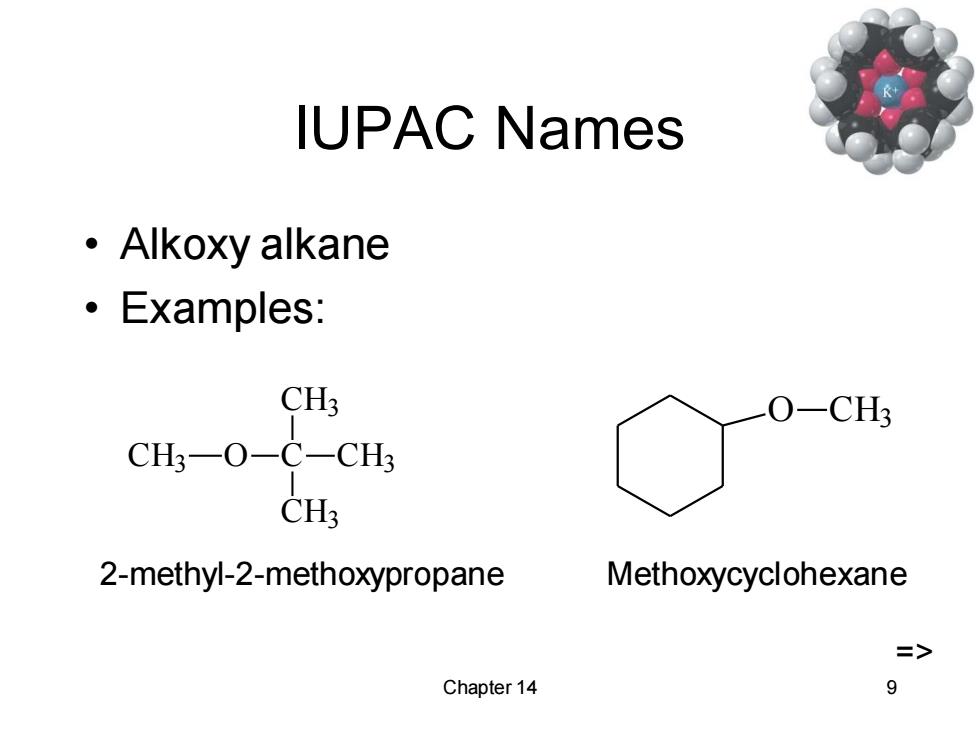
IUPAC Names ·Alkoxy alkane ·Examples: CH3 O-CH? CH3-O-C-CH3 CH3 2-methyl-2-methoxypropane Methoxycyclohexane => Chapter 14 9
Chapter 14 9 IUPAC Names • Alkoxy alkane • Examples: CH3 O C CH3 CH3 CH3 2-methyl-2-methoxypropane O CH3 Methoxycyclohexane =>
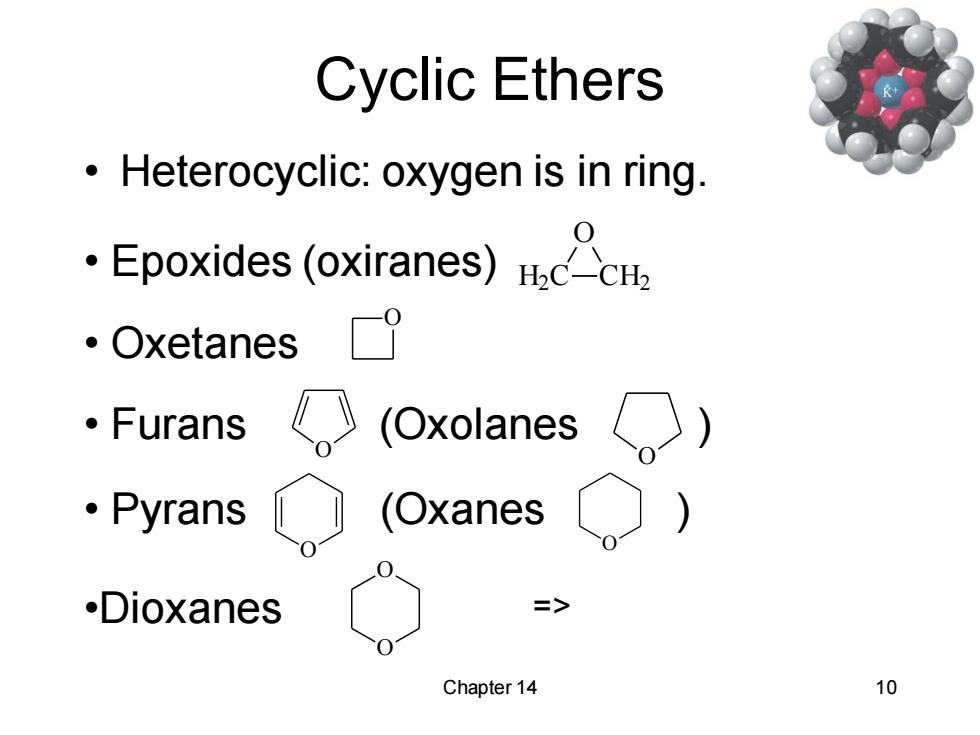
Cyclic Ethers Heterocyclic:oxygen is in ring Epoxides (oxiranes)c 。Oxetanes 。Furans 《(Oxolanes ·Pyrans (Oxanes .Dioxanes > Chapter 14 10
Chapter 14 10 Cyclic Ethers • Heterocyclic: oxygen is in ring. • Epoxides (oxiranes) H2C CH2 O • Oxetanes O • Furans (Oxolanes ) O O • Pyrans (Oxanes ) O O •Dioxanes O O =>