
Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 23 Carbohydrates and Nucleic Acids Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2006,Prentice Hall
Chapter 23 Carbohydrates and Nucleic Acids Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2006, Prentice Hall Organic Chemistry, 6th Edition L. G. Wade, Jr
Carbohydrates Synthesized by plants using sunlight to convert CO2 and H2O to glucose and O2. Polymers include starch and cellulose. Starch is storage unit for solar energy. Most sugars have formula C(H2O)n, “hydrate of carbon.” 二> Chapter 23 2
Chapter 23 2 Carbohydrates • Synthesized by plants using sunlight to convert CO2 and H2O to glucose and O2 . • Polymers include starch and cellulose. • Starch is storage unit for solar energy. • Most sugars have formula Cn (H2O)n , “hydrate of carbon.” =>

Classification of Carbohydrates Monosaccharides or simple sugars >polyhydroxyaldehydes or aldoses >polyhydroxyketones or ketoses Disaccharides can be hydrolyzed to two monosaccharides. Polysaccharides hydrolyze to many monosaccharide units.E.g.,starch and cellulose have 1000 glucose units. => Chapter 23 3
Chapter 23 3 Classification of Carbohydrates • Monosaccharides or simple sugars ➢polyhydroxyaldehydes or aldoses ➢polyhydroxyketones or ketoses • Disaccharides can be hydrolyzed to two monosaccharides. • Polysaccharides hydrolyze to many monosaccharide units. E.g., starch and cellulose have > 1000 glucose units. =>
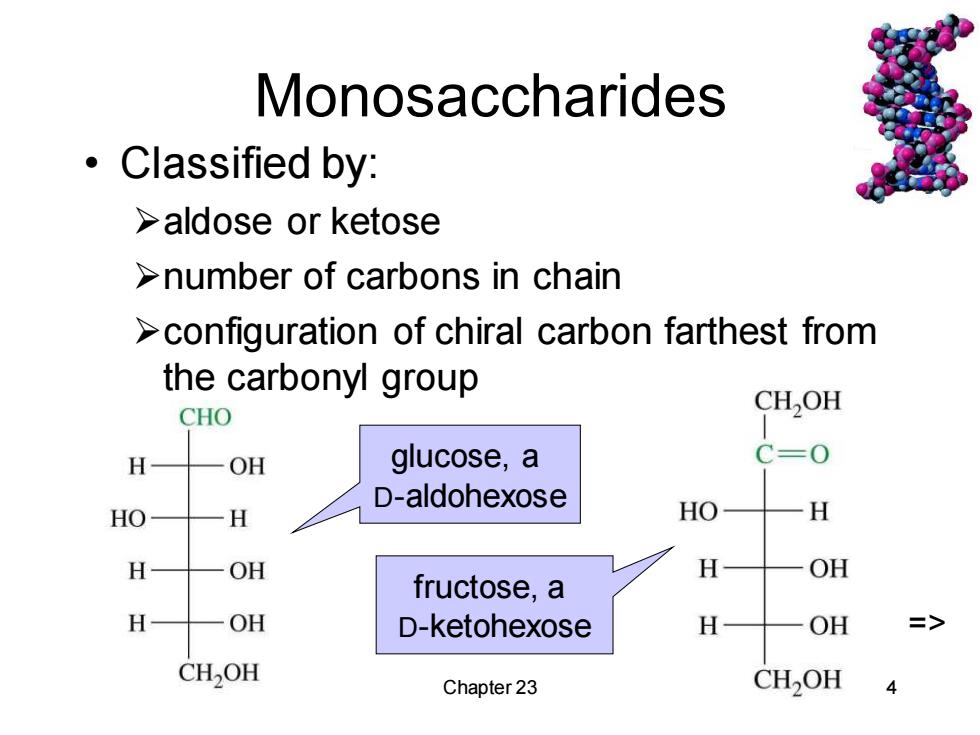
Monosaccharides Classified by: >aldose or ketose >number of carbons in chain >configuration of chiral carbon farthest from the carbonyl group CHO CH,OH 4 OH glucose,a C=0 D-aldohexose H HO H H OH H OH fructose,a H OH D-ketohexose H OH => CH2OH Chapter 23 CH2OH
Chapter 23 4 Monosaccharides • Classified by: ➢aldose or ketose ➢number of carbons in chain ➢configuration of chiral carbon farthest from the carbonyl group glucose, a D-aldohexose fructose, a D-ketohexose =>

D and L Sugars D sugars can be degraded to the dextrorotatory(+)form of glyceraldehyde. L sugars can be degraded to the levorotatory(-)form of glyceraldehyde. CHO C02↑ H一C一OH CHO C02↑ HO一C一H degrade HO一C一H degrade CHO CO2t H-C- OH H一C-OH H一C一OH CHO H-C-OH H-C-OH H-C-OH degrade H一C一OH CH2OH CH2OH CH2OH CH2OH D-(+)-glucose D-(-)-arabinose D-(-)-erythrose D-(+)-glyceraldehyde Copyright2005 Pearson Prentice Hall,Inc. => Chapter 23 5
Chapter 23 5 D and L Sugars • D sugars can be degraded to the dextrorotatory (+) form of glyceraldehyde. • L sugars can be degraded to the levorotatory (-) form of glyceraldehyde. =>
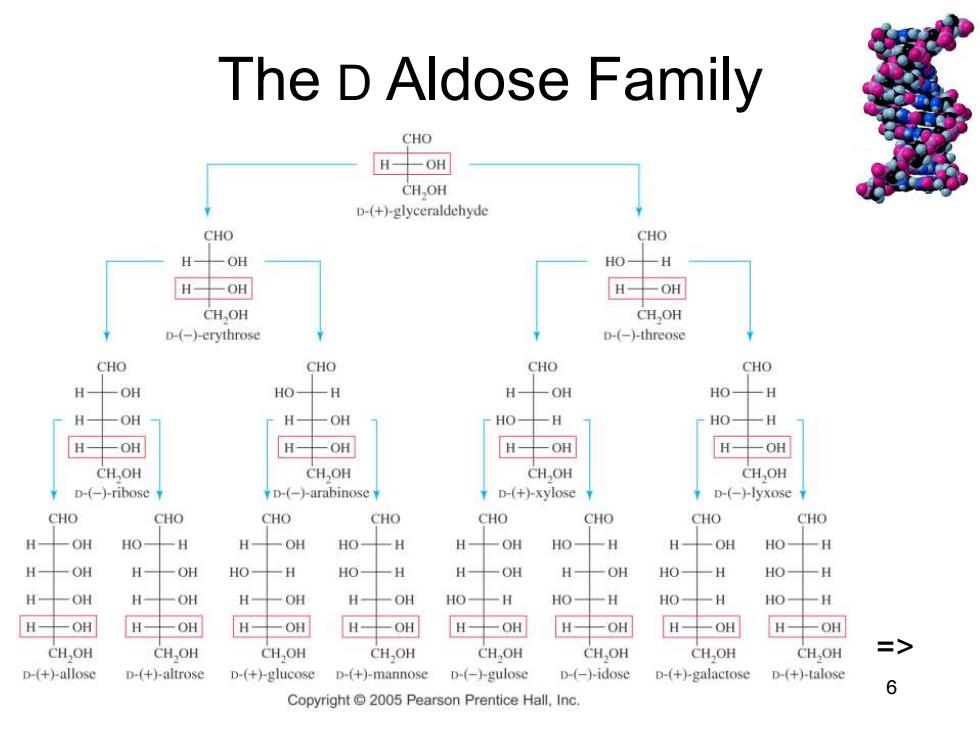
The D Aldose Family CHO H-OH CH.OH D-(+)-glyceraldehyde CHO CHO H-OH HO-H H-OH H-OH CH,OH CH,OH D-(-)-erythrose D-(-)-threose CHO CHO CHO CHO H -OH HO- H H-OH HO-H H -OH H--OH HOH HO-H H--OH H-OH H-OH H-OH CHOH CHOH CH,OH CH,OH D-(-)-ribose D-(-)-arabinose D-(+)-xylose D-(-)-lyxose CHO CHO CHO CHO CHO CHO CHO CHO H OH HO- H H 一OH HO- -H H- 一OH HO- H H -OH HOH H -OH H一OH HO-H HO- 一H H 一OH H -OH HO- H HO一H H -OH H 一OH H一OH H 一OH HO- H HO- 一H HO -H HO-H H-OH H一OH H-OH H-OH H-OH H-OH H一OH H-OH CH,OH CH.OH CHOH CH,OH CHOH CH,OH CH,OH CH,OH D-(+)-allose D-(+)-altrose D-(+)-glucose D-(+)-mannose D-(-)-gulose D-(-)-idose D-(+)-galactose D-(+)-talose Copyright2005 Pearson Prentice Hall.Inc. 6
Chapter 23 6 The D Aldose Family =>
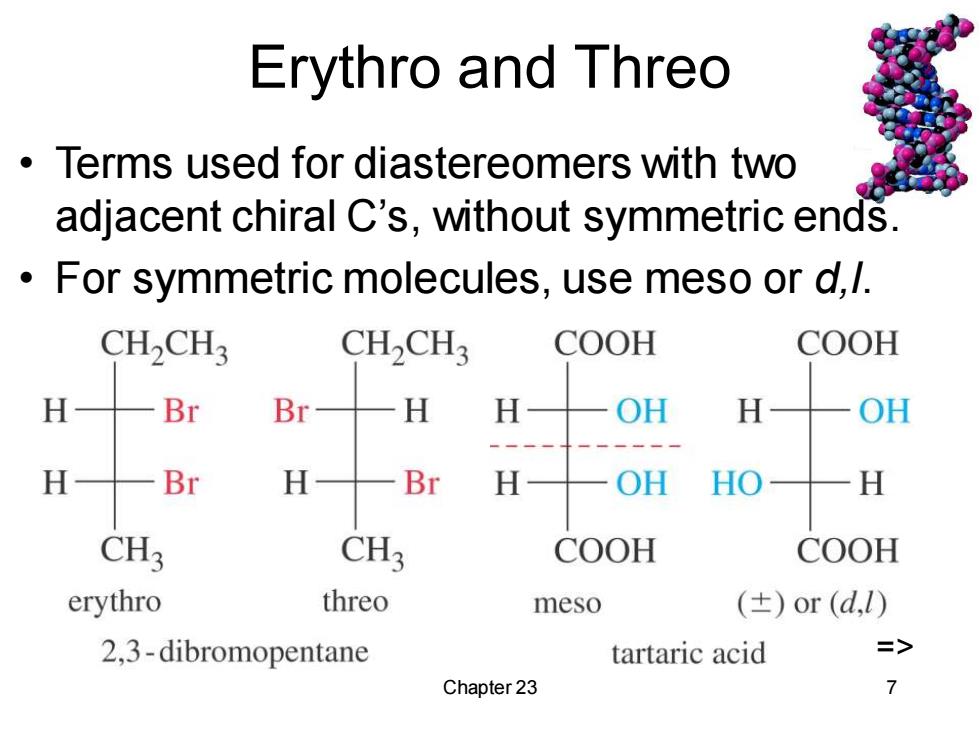
Erythro and Threo Terms used for diastereomers with two adjacent chiral C's,without symmetric ends. For symmetric molecules,use meso or d,1. CH2CH3 CH2CH3 COOH COOH H Br Br H H OH H OH H Br H Br H OH HO H CH3 CH3 COOH COOH erythro threo meso (±)or(d,) 2,3-dibromopentane tartaric acid => Chapter 23 7
Chapter 23 7 Erythro and Threo • Terms used for diastereomers with two adjacent chiral C’s, without symmetric ends. • For symmetric molecules, use meso or d,l. =>
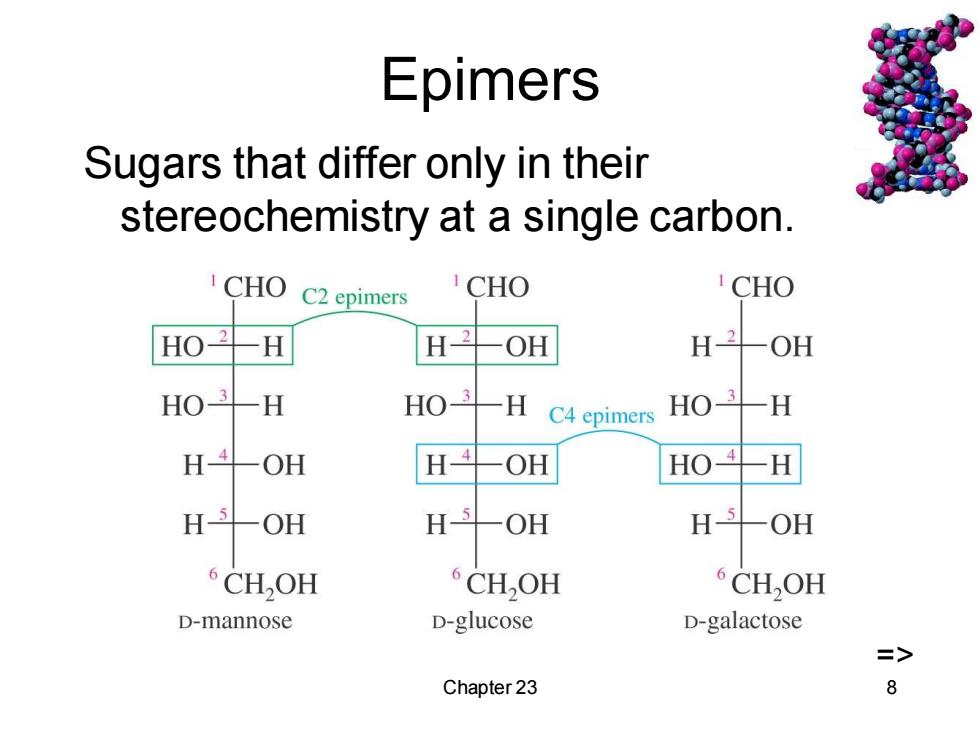
Epimers Sugars that differ only in their stereochemistry at a single carbon. CHO C2 epimers CHO CHO H OH H-2 OH HO -H HO3 -H C4 epimers HO-H H -OH H--OH HO4H H-5-OH H-5 OH H -OH CH,OH CH,OH CH,OH D-mannose D-glucose D-galactose => Chapter 23 8
Chapter 23 8 Epimers Sugars that differ only in their stereochemistry at a single carbon. =>
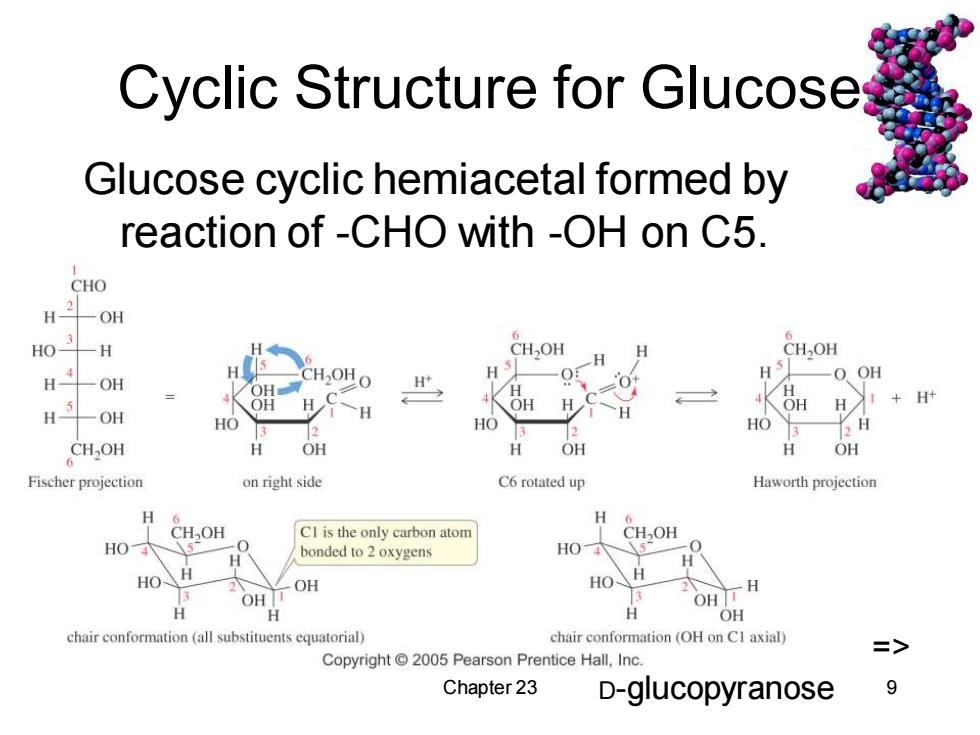
Cyclic Structure for Glucoses Glucose cyclic hemiacetal formed by reaction of -CHO with -OH on C5. CHO H3/ -OH -H CHOH H CHOH H H -O OH OH CH OH O H O+ H H OH H Hy+H 一OH H 4KòH HO 2 HO 3 H CH,OH OH H OH OH Fischer projection on right side C6 rotated up Haworth projection H 6 H CHOH CI is the only carbon atom CHOH HO bonded to 2 oxygens HO -0 HO H HO、 H 3 OH OH 0 OH TH H OH chair conformation (all substituents equatorial) chair conformation (OH on Cl axial) Copyright 2005 Pearson Prentice Hall,Inc. Chapter 23 D-glucopyranose 9
Chapter 23 9 Cyclic Structure for Glucose Glucose cyclic hemiacetal formed by reaction of -CHO with -OH on C5. => D-glucopyranose
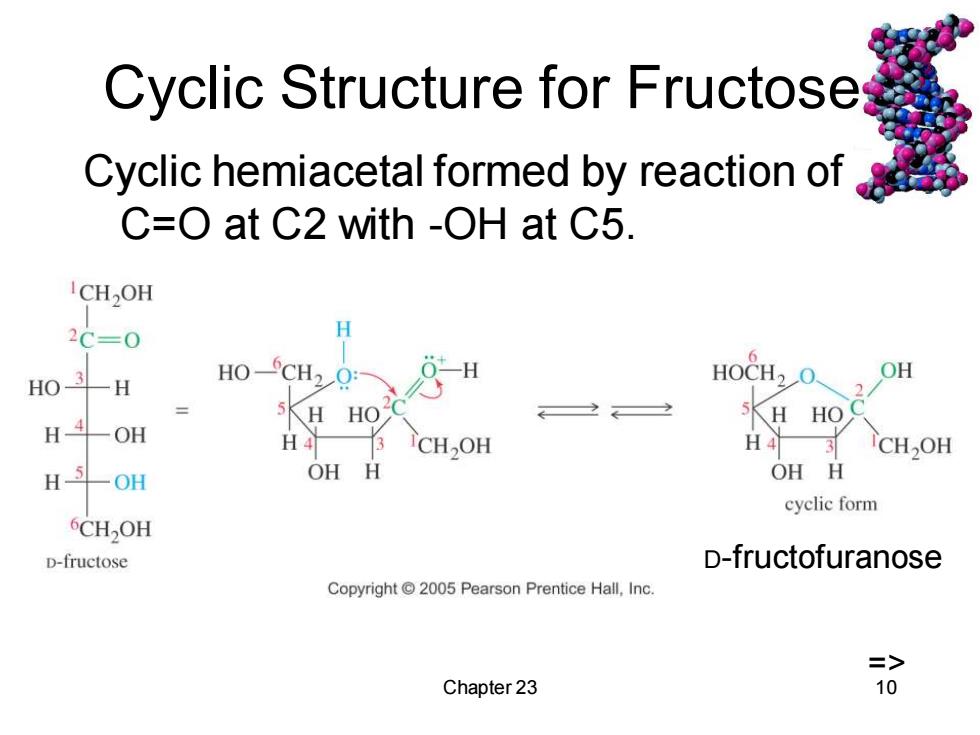
Cyclic Structure for Fructoses Cyclic hemiacetal formed by reaction of C=O at C2 with -OH at C5. CH2OH 2C=0 H HO3-H HO-CH2Q: -H HOCH2O OH HO H 5K HO 一OH H4 CH2OH H43 CH2OH H-5-OH OH H OH H cyclic form 6CHOH D-fructose D-fructofuranose Copyright 2005 Pearson Prentice Hall,Inc. => Chapter 23 10
Chapter 23 10 Cyclic Structure for Fructose Cyclic hemiacetal formed by reaction of C=O at C2 with -OH at C5. => D-fructofuranose