
Organic Compounds: Alkanes and Cycloalkanes Based on McMurry's Organic Chemistry,6th edition,Chapter 3
Organic Compounds: Alkanes and Cycloalkanes Based on McMurry’s Organic Chemistry, 6th edition, Chapter 3
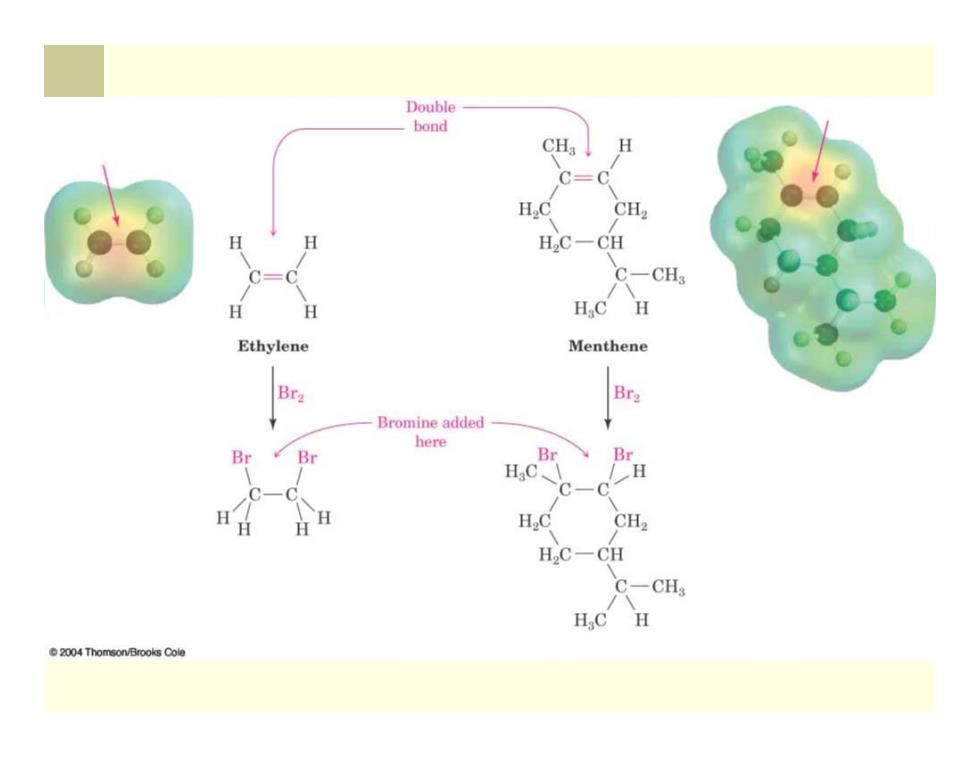
Families of Organic Compounds Organic compounds can be grouped into families by their common structural features The common structural feature that makes it possible to classify compounds by reactivity are called functional groups Functional group-collection of atoms at a site within a molecule with a common bonding pattern The group reacts in a typical way,generally independent of the rest of the molecule For example,the double bonds in simple and complex alkenes react with bromine in the same way
Families of Organic Compounds Organic compounds can be grouped into families by their common structural features The common structural feature that makes it possible to classify compounds by reactivity are called functional groups Functional group - collection of atoms at a site within a molecule with a common bonding pattern The group reacts in a typical way, generally independent of the rest of the molecule For example, the double bonds in simple and complex alkenes react with bromine in the same way
Double bond CH C=C CH2 H2C一CH C-CH3 H H HC H Ethylene Menthene Br2 Br2 Bromine added here Br Br -H C-C H.C CH2 HC一CH C-CH HC 日 2004 Thomson/Brooks Cole
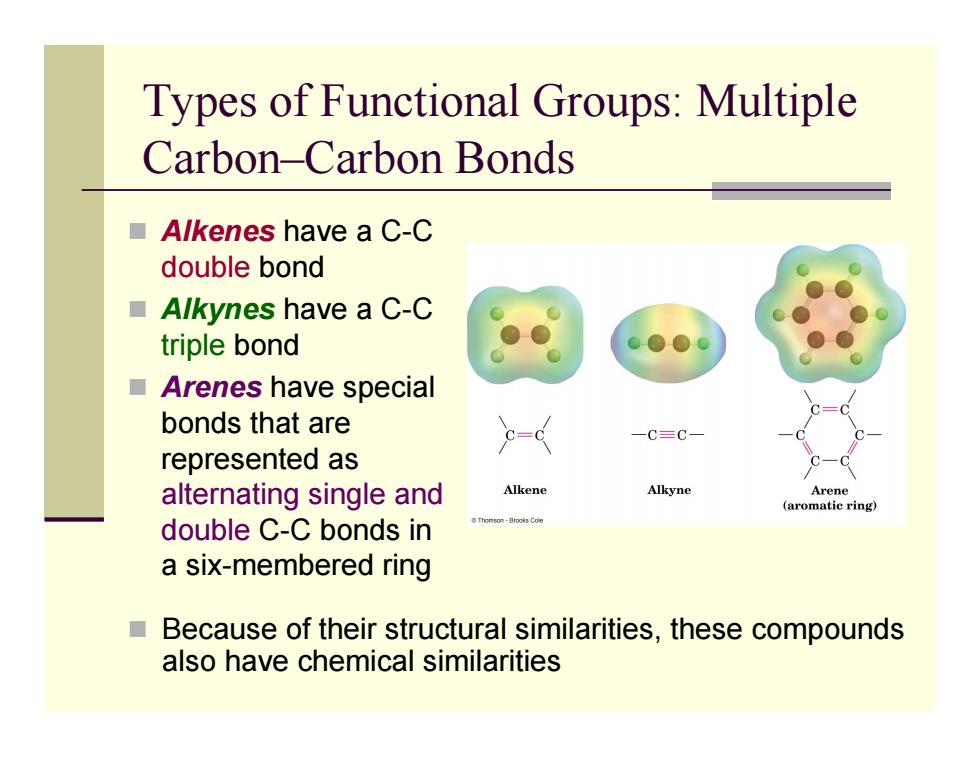
Types of Functional Groups:Multiple Carbon-Carbon Bonds ■Alkenes have a C-C double bond ■Alkynes have a C-C triple bond ■Arenes have special bonds that are 一C三C represented as alternating single and Alkene Alkyne Arene (aromatic ring) double C-C bonds in a six-membered ring Because of their structural similarities,these compounds also have chemical similarities
Types of Functional Groups: Multiple Carbon–Carbon Bonds Alkenes have a C-C double bond Alkynes have a C-C triple bond Arenes have special bonds that are represented as alternating single and double C-C bonds in a six-membered ring Because of their structural similarities, these compounds also have chemical similarities

Functional Groups with Carbon Singly Bonded to an Electronegative Atom Alkyl halide:C bonded to halogen (C-X) Alcohol:C bonded O of a hydroxyl group(C-OH) Ether:Two C's bonded to the same O(C-O-C) Amine:C bonded to N(C-N) ■ Thiol:C bonded to SH group(C-SH) ■ Sulfide:Two C's bonded to same S(C-S-C) Bonds are polar,with partial positive charge on C (+)and partial negative charge(⑧-)on electronegative atom
Functional Groups with Carbon Singly Bonded to an Electronegative Atom Alkyl halide: C bonded to halogen (C-X ) Alcohol: C bonded O of a hydroxyl group ( C OH ) Ether: Two C’s bonded to the same O ( C O C ) Amine : C bonded to N ( C N ) Thiol : C bonded to SH group ( C SH ) Sulfide: Two C’s bonded to same S ( C S C ) Bonds are polar, with partial positive charge on C ( δ+) and partial negative charge (δ−) on electronegative atom
Groups with a Carbon-Oxygen Double Bond (Carbonyl Groups) Aldehyde:one hydrogen bonded to C=O Ketone:two C's bonded to the C=O Carboxylic acid:-OH bonded to the C=O Ester:C-O bonded to the C=O Amide:C-N bonded to the C=O Acid chloride:CI bonded to the C=O ■Carbonyl C has partial positive charge(δ+) Carbonyl O has partial negative charge(5-)
Groups with a Carbon–Oxygen Double Bond (Carbonyl Groups) Aldehyde: one hydrogen bonded to C=O Ketone: two C’s bonded to the C=O Carboxylic acid: ⎯OH bonded to the C=O Ester: C-O bonded to the C=O Amide: C-N bonded to the C=O Acid chloride: Cl bonded to the C=O Carbonyl C has partial positive charge ( δ+) Carbonyl O has partial negative charge ( δ-)
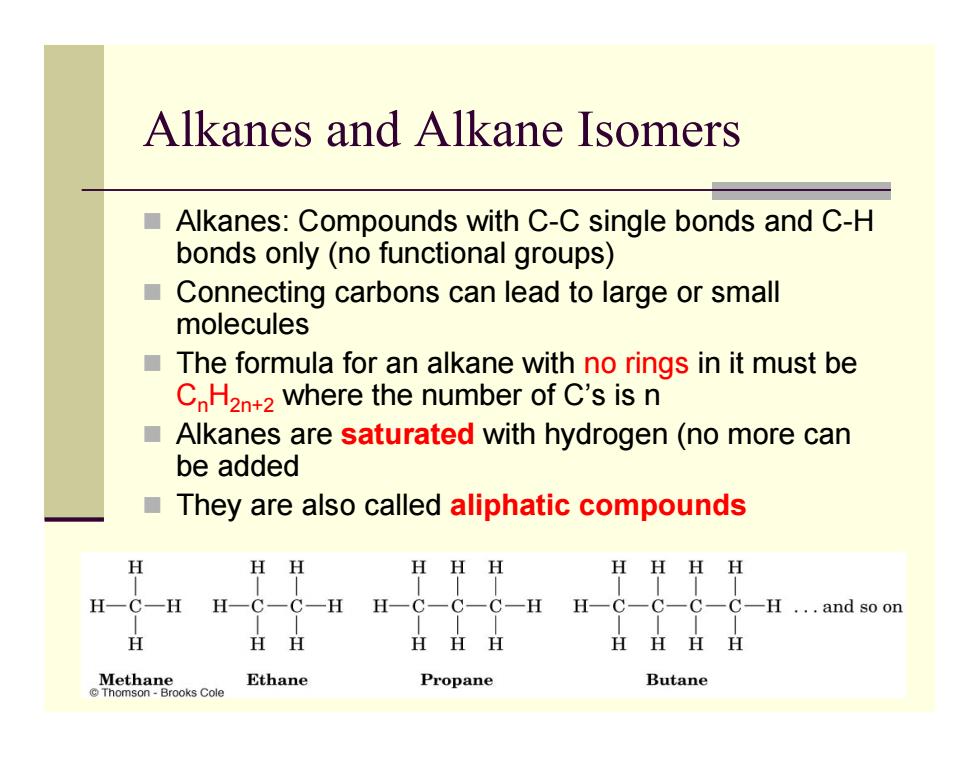
Alkanes and Alkane Isomers Alkanes:Compounds with C-C single bonds and C-H bonds only(no functional groups) ■ Connecting carbons can lead to large or small molecules The formula for an alkane with no rings in it must be C H2n+2 where the number of C's is n ■ Alkanes are saturated with hydrogen(no more can be added They are also called aliphatic compounds HH HHH HHHH H一C-H H-C-C-H H-C-C-C-H H-C-C-C-C-H ...and so on H HH HHH H丑HH Methaneosca Ethane Propane Butane
Alkanes and Alkane Isomers Alkanes: Compounds with C-C single bonds and C-H bonds only (no functional groups) Connecting carbons can lead to large or small molecules The formula for an alkane with no rings in it must be C n H2n+2 where the number of C’s is n Alkanes are saturated with hydrogen (no more can be added They are also called aliphatic compounds
Alkane Isomers ■ CH4=methane,C2H6=ethane,C3H8=propane The molecular formula of an alkane with more than three carbons can give more than one structure Ca(butane)=butane and isobutane Cs(pentane)=pentane,2-methylbutane,and 2,2- dimethylpropane Alkanes with C's connected to no more than 2 other C's are straight-chain or normal alkanes Alkanes with one or more C's connected to 3 or 4 C's are branched-chain alkanes
Alkane Isomers CH 4 = methane, C 2 H 6 = ethane, C 3 H 8= propane The molecular formula of an alkane with more than three carbons can give more than one structure C4 (butane) = butane and isobutane C5 (pentane) = pentane, 2-methylbutane, and 2,2- dimethylpropane Alkanes with C’s connected to no more than 2 other C’s are straight-chain or normal alkanes Alkanes with one or more C’s connected to 3 or 4 C’s are branched-chain alkanes
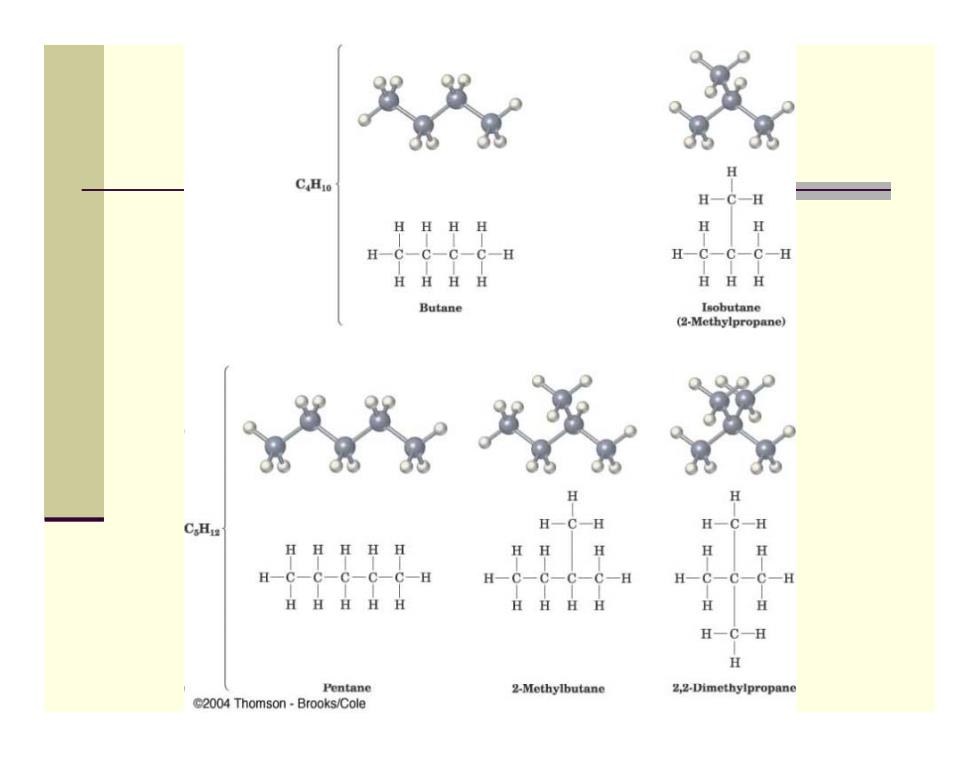
H-C一C-C一H HHH Butane Isobutane (2-Methylpropane) C.Hiz H H H H一C H Pentane 2-Methylbutane 2,2-Dimethylpropane 2004 Thomson Brooks/Cole

Constitutional Isomers ■ Isomers that differ in how their atoms are arranged in chains are called constitutional isomers ■ Compounds other than alkanes can be constitutional isomers of one another They must have the same molecular formula to be isomers Different carbon CH3 skeletons C,H10 CH CHCH and CH CH2CH2CHa 2-Methylpropane Butane (Isobutane) Different functional CHCH,OH and CHOCH3 groups C.HO Ethyl alcohol Dimethyl ether Different position of NH2 functional groups C.HoN CH CHCH3 and CH:CH2CH2NH2 Isopropylamine Propvlamine Thomson-Brooks Cole
Constitutional Isomers Isomers that differ in how their atoms are arranged in chains are called constitutional isomers Compounds other than alkanes can be constitutional isomers of one another They must have the same molecular formula to be isomers