Structure and Bonding Based on McMurry's Organic Chemistry,6th edition, Chapter 1
Structure and Bonding Based on McMurry’s Organic Chemistry, 6th edition, Chapter 1

Organic Chemistry A study of carbon compounds H He Be B Ne Na Mg Al Si Ar Sc Ti Cr Mn Fe Co Ni Cu Ga Ge Se Br Kr Rb Sr Zr Nb Sn Xe Cs Ba La Hf Ta Re Os Ir Au Hg T Pb Bi Po At Rn Fr Ra Ac Rf Db Sg Bh Hs Mt 2004 Thon Although C is the principal element in organic compounds,most also contain H,and many contain N,O,P,S.CI or other elements
Organic Chemistry A study of carbon compounds Although C is the principal element in organic compounds, most also contain H, and many contain N, O, P, S. Cl or other elements
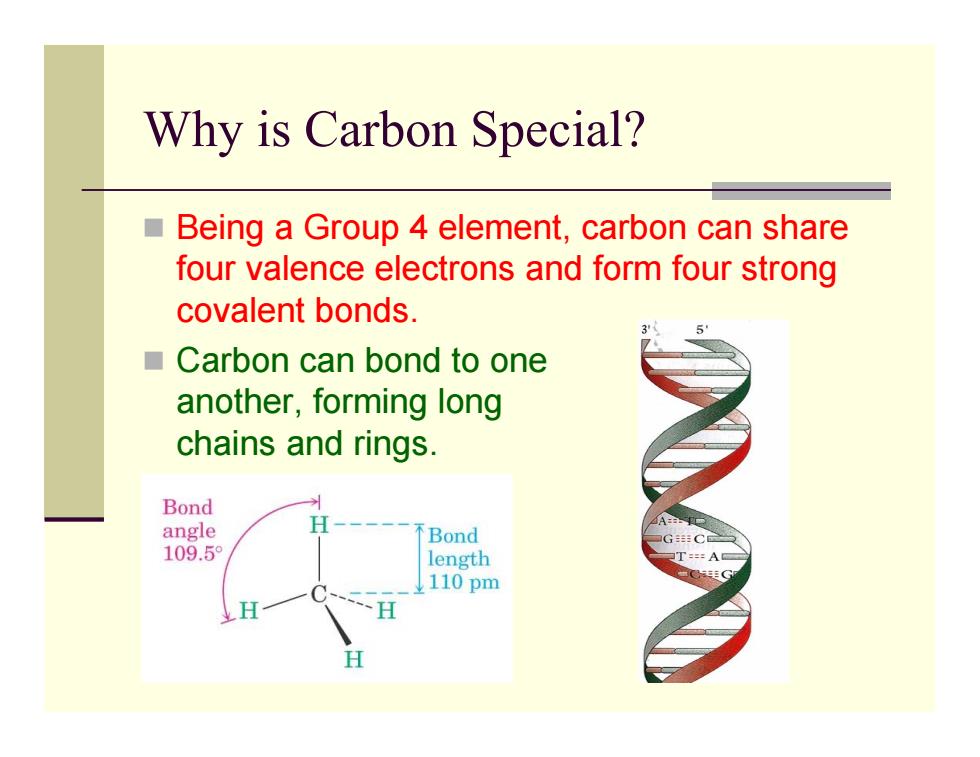
Why is Carbon Special? ■ Being a Group 4 element,carbon can share four valence electrons and form four strong covalent bonds. ■Carbon can bond to one another,forming long chains and rings. Bond angle Bond GC 109.5 length 110pm H
Why is Carbon Special? Being a Group 4 element, carbon can share four valence electrons and form four strong covalent bonds. Carbon can bond to one another, forming long chains and rings
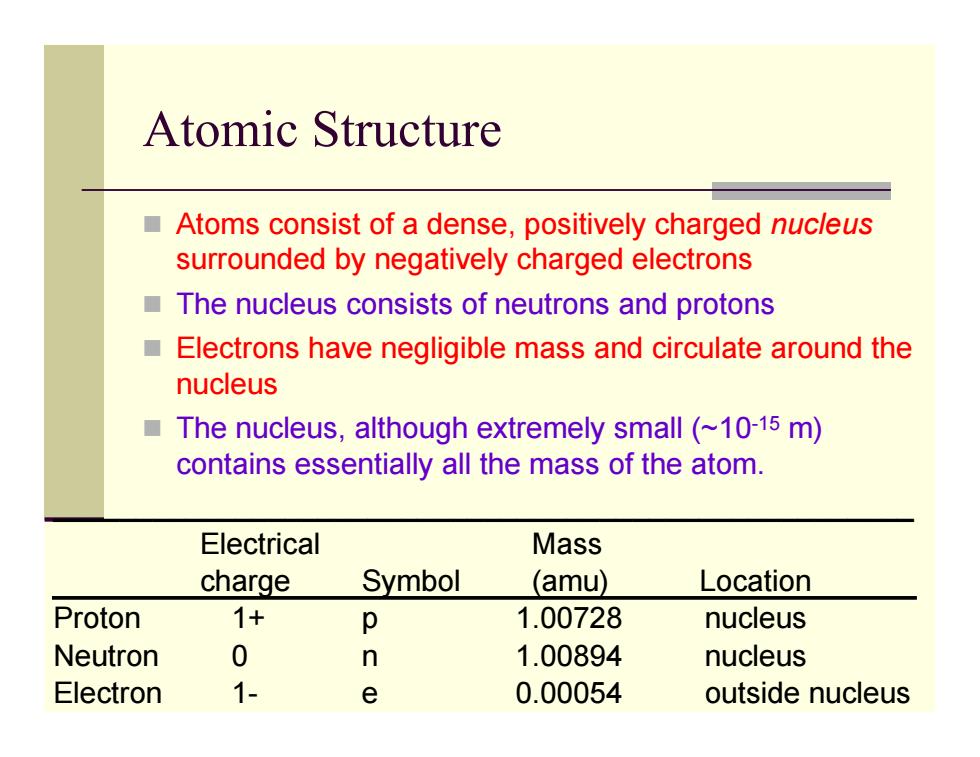
Atomic Structure Atoms consist of a dense,positively charged nucleus surrounded by negatively charged electrons ■ The nucleus consists of neutrons and protons ■ Electrons have negligible mass and circulate around the nucleus ■ The nucleus,although extremely small(~10-15 m) contains essentially all the mass of the atom. Electrical Mass charge Symbol (amu) Location Proton 1+ p 1.00728 nucleus Neutron 0 n 1.00894 nucleus Electron 1- e 0.00054 outside nucleus
Atomic Structure Atoms consist of a dense, positively charged nucleus surrounded by negatively charged electrons The nucleus consists of neutrons and protons Electrons have negligible mass and circulate around the nucleus The nucleus, although extremely small (~10-15 m) contains essentially all the mass of the atom. ____________________________________________________ Electrical Mass charge Symbol (amu) Location Proton 1+ p 1.00728 nucleus Neutron 0 n 1.00894 nucleus Electron 1- e 0.00054 outside nucleus

Atomic Number and Atomic Mass The atomic number(Z)is the number of protons in the atom's nucleus The mass number (A)is the number of protons plus neutrons All the atoms of a given element have the same atomic number ■ Isotopes are atoms of the same element that have different numbers of neutrons and therefore different mass numbers The atomic mass of an element is the weighted average mass in atomic mass units (amu)of an element's naturally occurring isotopes
Atomic Number and Atomic Mass The atomic number ( Z) is the number of protons in the atom's nucleus The mass number ( A) is the number of protons plus neutrons All the atoms of a given element have the same atomic number Isotopes are atoms of the same element that have different numbers of neutrons and therefore different mass numbers The atomic mass of an element is the weighted average mass in atomic mass units (amu) of an element’s naturally occurring isotopes

Atomic Structure:Orbitals How are the electrons distributed in an atom? ■ Quantum mechanics:behavior of a specific electron in an atoms is descrbied by a mathematical expression called a wave equation The solution of the wave equation is called an orbital An orbital describes the volume of space around a nucleus where an electron is mostly likely to be found
Atomic Structure: Orbitals Quantum mechanics: behavior of a specific electron in an atoms is descrbied by a mathematical expression called a wave equation The solution of the wave equation is called an orbital An orbital describes the volume of space around a nucleus where an electron is mostly likely to be found How are the electrons distributed in an atom?

Shapes of Atomic Orbitals Four different kinds of orbitals ■Denoted s,p,d,andf s and p orbitals most important in organic chemistry s orbitals:spherical,nucleus at center p orbitals:dumbbell-shaped,nucleus at middle An s orbital A p orbital A d orbital
Shapes of Atomic Orbitals Four different kinds of orbitals Denoted s, p, d, and f s and p orbitals most important in organic chemistry s orbitals: spherical, nucleus at center p orbitals: dumbbell-shaped, nucleus at middle

p-Orbitals ■The three perpendicular p orbitals,px,py,and pz,are of equal energy A 2px orbital A 2py orbital ■Lobes of a p orbital are separated by region of zero electron density,a node Thomson-Brooks Col A 2pz orbital Three 2p orbitals
p-Orbitals The three perpendicular p orbitals, p x, p y, and p z, are of equal energy Lobes of a p orbital are separated by region of zero electron density, a node

Orbitals and Shells ■ Orbitals are organized into shells of increasing size and energy ■ Different shells contain different numbers and kinds of orbitals Each orbital can be occupied by two electrons First shell contains one s orbital,denoted 1s ■ Second shell contains one s orbital(2s)and three p orbitals(2p) Third shell contains an s orbital(3s),three p orbitals (3p),and five d orbitals (3d)
Orbitals and Shells Orbitals are organized into shells of increasing size and energy Different shells contain different numbers and kinds of orbitals Each orbital can be occupied by two electrons First shell contains one s orbital, denoted 1 s Second shell contains one s orbital ( 2 s) and three p orbitals ( 2 p ) Third shell contains an s orbital ( 3 s), three p orbitals ( 3 p), and five d orbitals (3 d )
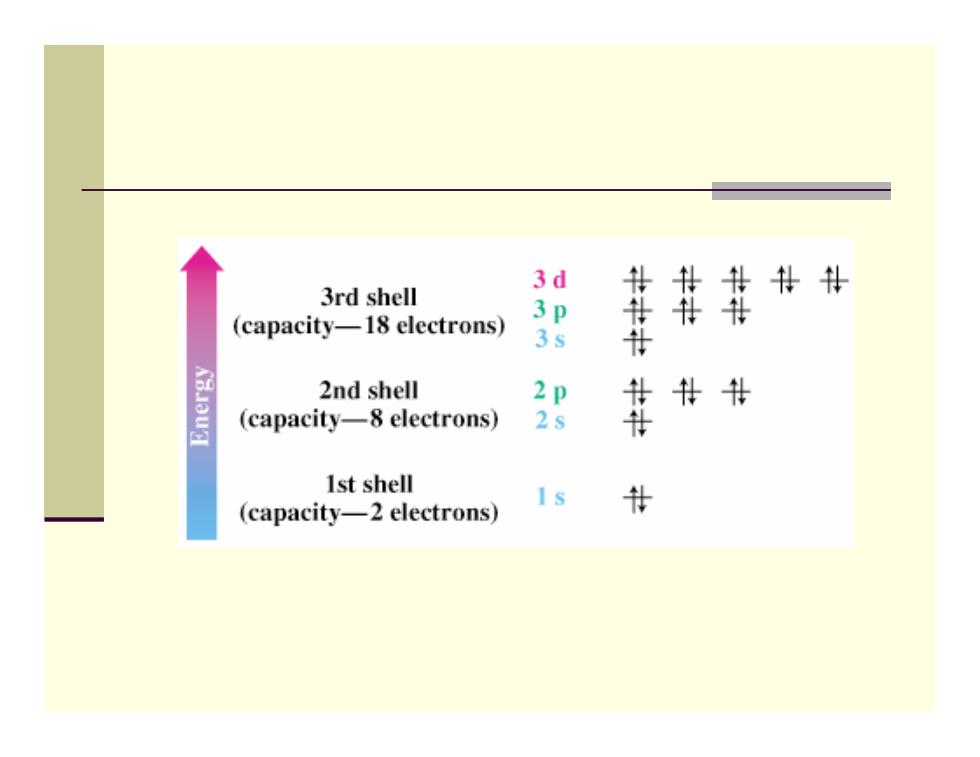
3d 3rd shell # (capacity-18 electrons) 3s 2nd shell 2 (capacity-8 electrons) 2s 鞋 1st shell (capacity-2 electrons) #